Diabetes mellitus is an important cause of death, illness and disability in Canada. It affects approximately 5% of adults, which equates to over one million Canadians1,2. Having diabetes substantially increases one's risk of developing blindness, end stage renal disease, lower limb amputations, and dying from coronary artery disease, stroke, or peripheral vascular disease3,4. Recent studies have shown that keeping blood sugar levels within normal range reduces chances of developing at least some of the complications associated with having diabetes5-7.
Consequently, an important aspect of type 2 diabetes treatment is lowering blood sugar levels through diet, exercise, and medications8-12. Comprehensive management of diabetic patients also includes monitoring blood pressure and lipids, encouraging smoking cessation, and the prophylactic use of acetylsalicylic acid (ASA)2. According to practice guidelines, initial and ongoing education of the patient with diabetes should be an integral part of diabetes management and not merely an adjunct to treatment. Whenever possible, diabetic patients should receive dietary advice from a registered dietician1,2,13. In addition to these recommendations, Aboriginal people have been singled out as deserving special attention - earlier screening and more aggressive medical management of relatively high blood pressure, blood glucose, and cholesterol levels2.
Type 2 diabetes has gone from being nearly nonexistent in the North American native Indian population in 1940 to current epidemic proportions14-19. According to a report published by Health Canada the prevalence rate of diabetes among the First Nations people in Canada is twice that of the general population of Canada17. Type 2 diabetes is of particular concern to Aboriginal people because of earlier onset, and higher rates of complications, co-morbidities, and diabetes related death seen in this population. Diabetes associated co-morbidities and complications which occur at rates higher than expected within the Aboriginal diabetic population include hypertension, obesity, cardiovascular disease, cerebrovascular disease, end-stage renal disease, lower leg amputations, and diabetic retinopathy, which all contribute to shortened life span reported for Aboriginal people16,18-21. For example, a study of diabetics living in Kahnawake (a Mohawk Indian community located near Montreal, Quebec) revealed that 13% had a history of stroke, versus 3% for the non-diabetic Kahnawake population; over half this diabetic population had coronary artery disease; 25% developed retinopathy after 10 years; and over 60% of this diabetic population had at least one major complication. The high rates of diabetes-related complications and co-morbidities have been attributed to earlier onset of disease coupled with a systemic failure to make the diagnosis in a timely fashion. According to the 2000/2001 Canadian Community Health Survey, Canadians living in rural and northern areas have higher rates of smoking, obesity, major depression, high blood pressure, and arthritis22. This is consistent with other studies that show rural residents have higher rates of chronic disease, rural residents report being ill more frequently, and they are more likely to report poorer health status23-26. Poorer health among rural residents has in turn been attributed to less education, lower income, and greater proportion of First Nations people in this population23,27-31. A possible factor is that rural residency is an important confounder in data reporting poor health for Aboriginal people; this needs to be examined more closely.
While there is quite a bit of information on health and prevalence of diabetes and other chronic disease for Canada's Aboriginal people, much of this information comes from self-reported data; in particular the 1991 Aboriginal Peoples Survey, and the 1997 First Nations and Inuit Regional health Survey which had a adequate total numbers, but relatively low regional response rates17,21. The community-based data which does exist, comes almost entirely from eastern Aboriginal communities. This is despite the fact that one-third of Canada's pre-contact Aboriginal population lived within British Columbia; and seven of 11 language families spoken within Canada were being spoken in British Columbia at the time of European contact32. Diabetes prevalence data from British Columbia suggests diabetes prevalence rates in this province for Aboriginal people are among the lowest in all of Canada18,33-35. A 1990 study showed that the prevalence rates of diabetes varied greatly among the provinces and territories, with the Atlantic Provinces and Ontario having the highest rates, at 8.7% and 7.6%, respectively, while British Columbia, the Yukon and Northwest Territories had the lowest, at 1.6%, 1.2% and 0.8% respectively33. While the overall prevalence rate for diabetes in British Columbia is relatively low, recent studies demonstrate significant regional differences within the province34,35.
Bella Coola Valley is a remote community located in the central coast region of British Columbia, Canada (Fig 1)36. While Bella Coola and Hagensborg are the two largest communities in this valley, there are a few houses in Stuie, and a few seasonal cabins located in Atnarko (Fig 1). According to the 2001 Census, 2285 people live in the Bella Coola Valley, and 46% of these people are of Aboriginal descent37,38. Bella Coola Valley is part of the traditional territory of the Nuxalk Nation, a tribe of Salish-speaking Coastal Indians39-41.
Figure 1: Map of the study area.
The crude prevalence of diabetes in the Aboriginal population is more than one and half times that of the non-Aboriginal population (9.7% vs 5.7%). However, when the differences in the age distribution of the Aboriginal and non-Aboriginal populations are considered, and the prevalence rates are adjusted, the prevalence of diabetes in the Aboriginal population is more than two and half times that of the non-Aboriginal population (12.5% vs 4.8%)42. Age, weight, Aboriginal status are all associated with having diabetes in this population42. An individual's sex was not associated with having diabetes in either the Aboriginal or non-Aboriginal population.
The research question of this study is: are there differences in diabetes related co-morbidity (eg, eye, kidney, heart, vascular, neurologic) prevalence rates between Aboriginal and non-Aboriginal diabetics living in the Bella Coola Valley, Canada?
Methods
This research project has been carried out in a participatory fashion, following the recommendations outlined in a recently published policy statement entitled 'A Guide for Health Professionals Working with Aboriginal Peoples'43-45. There was consultation with the Nuxalk Band Council, community members and local health care providers on our plans to study determinants of health and disease of people living in the Bella Coola valley. Prior to collecting data we obtained letters of support from the Nuxalk Band Council, from the Bella Coola Transitional Health Authority, and from Central Coast Regional District. Ethics approval to collect data was obtained from Research Ethics Committees located at both the University of British Columbia, and at the University of Northern British Columbia. The results and the manuscript were reviewed and approved for publication by both Nuxalk Health professionals and United Church Health Services health professionals.
Aboriginal status for the study population was determined from multiple sources: Nuxalk Band lists, a locally available genealogy, clinic chart, and a recent survey46. It was found that 47% of the people living in the Bella Coola Valley were of Aboriginal decent which is similar to the 2001 census estimate (46%)37,38. The total clinic population was 2376 people, which is slightly higher than the 2001 Census estimate. Of the 2376 clinic population, 1723 were adults (>18 years old), and 126 of these adults had diabetes mellitus. All 126 diabetics had been classified as having type 2 diabetes. At the time of the study, there was only one type 1 diabetic living in the Bella Coola Valley and that person was less than 18 years of age when the data for this study was collected. The presence of diabetes was based on a physician diagnosis of diabetes which, in turn, was based on the 1998 clinical practice guidelines for the management of diabetes in Canada2,47. The presence or absence of a variety of chronic diseases/co-morbidities was also noted during the chart review. Whether or not the adult smoked, was a former smoker, or had ever smoked was also noted, but the accuracy of these data was uncertain because the clinic has never made a concerted effort to record smoking status of its patients; also, the authors could not be certain whether a subject was a former smoker if the chart note simply stated 'not smoking'.
Chart-derived information was entered into an electronic EXCEL spreadsheet from which results were summarized, and graphs created; the data were sent to statisticians and other researchers for further analyses48. The data were analyzed using the software SPSS (SPSS Inc, Chicago, IL, USA) for Windows. Differences in the prevalence of chronic disease/co-morbidities between ethnic groups (Aboriginal vs others), and between people with or without diabetes were evaluated using Pearson's c2 test49.
Results
The average age of the adult (>18 years old) diabetic population (n = 126) was 60.2 + 12.3 (SD) years, and the average age of the non-diabetic adult (>18 years old) population (n = 1597) was 43.4 + 15.9 years.
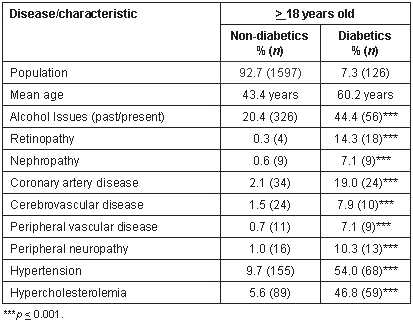
The prevalence of various co-morbidities in the >18 year old diabetic and non-diabetic populations (Table 1). Having a diagnosis of diabetes was clearly associated with increased prevalence of many different co-morbidities including history of alcohol issues, retinopathy, renal disease, coronary artery disease, cerebrovascular disease, peripheral vascular disease, peripheral neuropathy, hypertension and hypercholesterolemia.
Table 1: Prevalence of co-morbidity/chronic disease by age and diabetic status
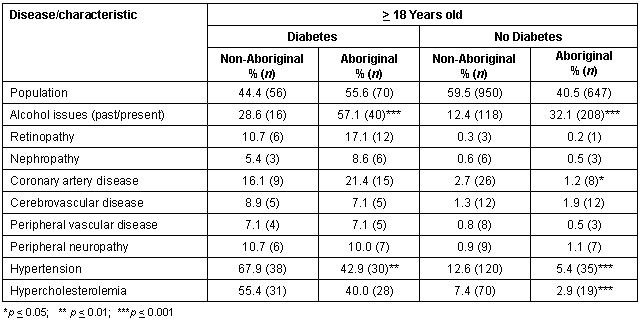
Stratifying by diabetic status and then comparing co-morbidity rates revealed a greater proportion of Aboriginal people had a history of alcohol issues whether they were diabetic or not; a greater proportion of non-Aboriginal adults had hypertension whether they were diabetic or not; and a greater proportion of non-Aboriginal non-diabetics had hypercholesterolemia compared with Aboriginal non-diabetics (Table 2).
Table 2: Co-morbidity stratified by diabetic status for Aboriginals and non-Aboriginals
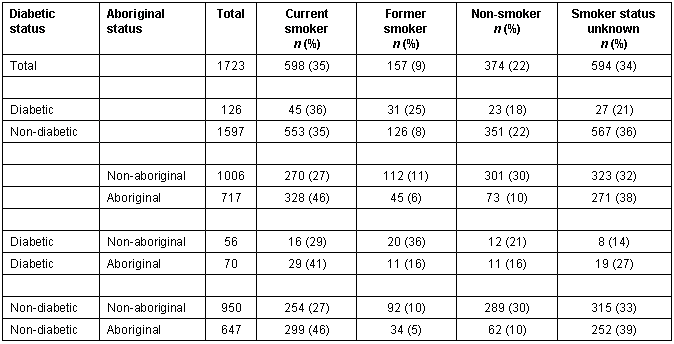
Smoking status of the clinic population is summarized (Table 3). At least 35% of Bella Coola adults smoked tobacco. Having diabetes did not alter this smoking rate much. Proportionately, more Aboriginal people smoked compared with non-Aboriginal people. Smoking status could not be determined in 34% of the charts reviewed.
Table 3: Smoking Status of Bella Coola Valley adults
Discussion
The information presented in this paper contributes to the scant knowledge which currently exists on the topic of Aboriginal diabetes related complications/co-morbidities for specific Western Aboriginal populations/communities43. The findings support previous literature which suggested diabetes is strongly associated with co-morbidities such as hypertension, hypercholesterolemia, and multiple end-organ diseases, for example coronary artery disease, retinopathy, nephropathy, and peripheral vascular disease.
The First Nations and Inuit Regional Health Survey (FNIHS) reported that diabetes, heart problems, and hypertension rates for First Nations people living on reserve were all increased up to three-fold, compared with the general Canadian population20,21.
Other studies have shown that rates of cerebrovascular disease, limb amputations, nephropathy, and retinopathy are all increased in Aboriginals, compared with the general Canadian population.
In the Bella Coola Valley, diabetes but not heart disease or hypertension prevalence is increased in the Aboriginal population. For example, the FNIHS reported 48% of Aboriginal diabetics had hypertension. In the Bella Coola Valley 43%, 68%, 5% and 13% of Aboriginal diabetics, non-Aboriginal diabetics, Aboriginal non-diabetics, and non-Aboriginal non-diabetics, respectively had a diagnosis of hypertension. A recent review of deaths in the Bella Coola Valley and Bella Coola Valley Health Area (LHA 49) between the years 1986 and 2001, revealed that compared with British Columbia non-Status Indian population, the standardized mortality ratio (SMR) for both Status Indian and residents who were not Status Indian was 1.4. The SMR for Status Indian residents living in the Bella Coola Valley dying from diabetes, circulatory diseases, ischemic heart disease, and cerebrovascular disease was 1.1, 0.9, 1.0 and 0.8 respectively. The SMR for residents who were not Status Indian for deaths due to diabetes, circulatory diseases, ischemic heart disease, and cerebrovascular disease was 0.9, 1.5, 1.4 and 2.6 respectively. None of the SMR reported for either Bella Coola Valley populations was statistically different from rates reported for the British Columbia non-Status Indian population50.
Failure to demonstrate increased diabetes or circulatory disease SMR, or increased prevalence rates of circulatory disease, ischemic heart disease, and cerebrovascular disease among the Bella Coola Aboriginal population is all the more surprising considering the fact that the smoking rate in this population exceeds the rate for non-Aboriginal Bella Coola Valley adult residents. Our chart-derived smoking rates were almost identical to those reported from a recent Bella Coola Valley health survey46. That survey reported a smoking rate of 34% for the entire adult population (Aboriginal and non-Aboriginal) and a smoking rate of 49% for the Bella Coola Valley Aboriginal adult population. According to our chart review, 35% of Bella Coola Valley adults currently smoke, and 46% of Aboriginal adults smoke. The aboriginal diabetic population has a smoking rate which exceeds 41%. Smoking rates in the Bella Coola Valley exceeds reported provincial and Canadian smoking rates22,46,51-52. Estimated smoking rate for Aboriginal people living in British Columbia51 is 43%, for the general British Columbia population51 it is 22%, and for the general Canadian population52 it is approximately 26%. According to the 2000/2001 Canadian Community health Survey, 33.5% of Canadians living in northern regions smoke and 32% of those in rural regions smoke22.
Salmon species (Onchorinchus sp.) and ooligans (Tahleichthys pacificus) are among the most heavily used food groups in the diet of Bella Coola Aboriginal residents. Perhaps the lower than expected cardiovascular disease rate is due to an above average consumption of dietary fish oils53-56. Dietary fish oils are rich in omega-3 polyunsaturated fatty acids which are believed to have numerous cardio-protective effects57. In 1985, Barr and Kuhnlein published information on high-density lipoprotein and total serum cholesterol levels for 202 members of the Nuxalk Nation and found that high density cholesterol levels were higher than expected53. They speculated that this finding might explain why British Columbia's west coast native people had lower coronary mortality rates than the general population at that time. A detailed study on the cholesterol profile in this population is indicated. Genetic differences may also contribute in some type of cardio-protective way in this Bella Coola Aboriginal population. This is also an area which warrants further investigation.
The finding that a history of alcohol abuse is strongly associated with the development of diabetes mellitus in both Aboriginal and non-Aboriginal populations raises the possibility that alcohol abuse is an under-appreciated contributor to high rates of diabetes reported in some Aboriginal populations58.
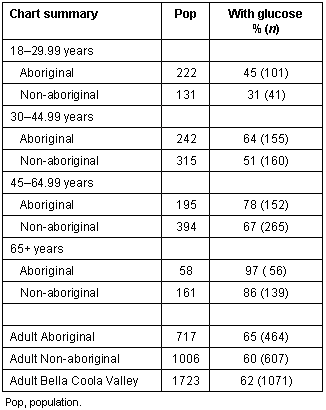
Findings such as ours could arise if the health care system preferentially screens and manages one population over another. Canada has a Universal Health Care system whereby access to hospital and medical services is considered to be a fundamental human right. In theory, the poorest Canadians should experience the same level of care as the wealthiest59. Our study populations were drawn from Bella Coola Valley residents who had been screened for non-insulin dependent diabetes mellitus. Although there was a push to screen all adults in this community for non-insulin dependent diabetes mellitus, this has not been successfully completed yet. The overall screening rate for diabetes in the adult Bella Coola Valley population is approximately 62%. The screening rate for adult Aboriginals is only slightly higher than that for adult non-Aboriginal people (65% vs 60%) which probably reflects the fact that current guidelines suggest Aboriginal people should be screened 5 to 10 years earlier than non-Aboriginal people1,2. As shown, older persons are much more likely to have a clinic chart blood glucose value than a younger person (Table 4).
Table 4: Percentage of charts with glucose screening
Co-morbid conditions such as obesity could account for some of the differences between Aboriginal and non-Aboriginal populations. We do know from another study that more Aboriginal (65%) than non-Aboriginal people (47%) living in the Bella Coola are overweight (defined as a BMI >27); and we do know that the proportion of population that were overweight increased with age42,60. However, logistic modeling confirms Aboriginal status is a risk factor for diabetes over above weight and age risk factors (Appendix I).
Our data suffer from limitations inherent in collecting medical chart information, especially incomplete information and non-standardized measurements. Reliability of the data could have been strengthened by having an independent review of a random sample of charts to assess for congruent findings among reviewers.
Conclusion
In summary, the present research provides baseline rates for diabetes related co-morbidity for the Aboriginal and non-Aboriginal peoples living in the Bella Coola Valley.
The development of diabetes in both Aboriginal and non-Aboriginal people living in the Bella Coola Valley is clearly associated with the presence of multiple co-morbidities including hypertension, hypercholesterolemia, coronary artery disease, cerebrovascular disease, nephropathy, and neuropathy. Surprisingly, we were unable to demonstrate that Aboriginal people living in the Bella Coola Valley have an increased prevalence of diabetes-associated co-morbidities over and above that found in the non-Aboriginal diabetic population. We speculate that a diet rich in fish oils (omega-3 fatty acids) may account for the lower than expected rates of cardiovascular disease.
Acknowledgements
The authors would like to thank Rhonda Elliott for her assistance with the chart review process. The authors would also like to acknowledge the support of Bill Tallio, Director of Nuxalk Wellness Program, and Dr R McIlwain, Director, United Church Health Services for their support of this project. UBC Family Practice Research Department; and UNBC provided research funding for this project. Dr Thommasen would like to acknowledge the Vancouver Foundation's Community-Based Clinician-Investigator Program for providing financial support.
References
1. Expert Committee of the Canadian Advisory Board. Clinical practice guidelines for the management of diabetes in Canada. Canada Medical Association Journal 1998; 159(8): S1-S29.
2. Canadian Diabetes Association. Canadian Diabetes Association 2003 Clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian Journal of Diabetes 2003; 27(2): S1-S152
3. Wilson PW, Cupples LA, Kannel WB. Is hyperglycemia associated with cardiovascular disease? Framingham Study. American Heart Journal 1991; 121: 586-90.
4. Murphy SL. Final data from 1998. National Vital Statistics Reports 2000: 48(11): 1-106. Available: http://www.cdc.gov/nchs/products/pubs/pubd/nvsr/nvsr.htm (Accessed 4 Oct 2004)
5. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993; 329: 977-986.
6. UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood Glucose Control with Sulphonylurea or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type II Diabetes (UKPDS 33). Lancet 1998; 352: 837-853.
7. United Kingdom Prospective Diabetes Study Group. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405-412.
8. Vaaler S. Optimal glycemic control in type II diabetic patients. Diabetes Care 2000; 23(2): B30-34.
9. Brown SA. Promoting weight loss in type II diabetes. Diabetes Care 1996; 19: 613-621.
10. Pan X, Li G. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. Diabetes Care 1997; 20: 537-543.
11. Schneider SH. Ten year experience with an exercise-based outpatient life-style modification program in the treatment of diabetes mellitus. Diabetes Care 1992; 15: 1800-1809.
12. Hu F, Stampfer MJ. Physical activity and risk of cardiovascular events in diabetic women. Annuals of Internal Medicine 2001; 134: 96-103.
13. American Diabetes Association: Clinical Practice Recommendations 2001. Diabetes Care 2001; 24: S80-S82.
14. Evers S, McCracken E, Antone I, Deagle G. The prevalence of diabetes in Indians and Caucasians living in southwestern Ontario. Canadian Journal of Public Health 1987; 78: 240-243.
15. Pioro MP, Dyck RF, Gillis DC. Diabetes prevalence rates among First Nations adults on Saskatchewan reserves in 1990: comparison by tribal grouping, geography and with non-First Nation people. Canadian Journal of Public Health 1996; 87(5): 325-328.
16. Macaulay AC, Montour LT, Adelson N. Prevalence of diabetic and atherosclerotic complications among Mohawk Indians of Kahnawake, PQ. Canadian Medical Association Journal 1988; 139: 221-224.
17. Bobet E. Diabetes among First Nations people. Health Canada cat. # H34-88/1998E. Ottawa: Minsitry of Public Works and Government Services Canada, 1998; 31.
18. Young TK. The health of Native Americans: towards a biocultural epidemiology. New York: Oxford University Press, 1994.
19. MacMillan HL, MacMillan AB, Offord DR, Dingle JL. Aboriginal health. Canida Medical Association Journal 1996; 155: 1569-1578
20. Health Canada. Diabetes in Canada, 2nd edn. Ottawa: Center for Chronic Disease Prevention and Control, Population and Public Health Branch, 2002.
21. First Nations and Inuit Regional Health Survey National Steering Committee. First Nations and Inuit Regional Health Survey National Report. No city: Assembly of First Nations Resource Centre, 1999.
22. Mitura V, Bollman RD. The health of rural Canadians: a rural-urban comparison of health indicators. Rural and small town Canada analysis bulletin. Statistics Canada 2003; 4(6): 1-23.
23. British Columbia Provincial Health Officer. A report on the health of British Columbians: Provincial Health Officer's annual report 1996. Victoria, BC: Ministry of Health and Ministry Responsible for Seniors, 1997.
24. Thiede-Call K, Casey M, Radcliff T. Rural beneficiaries with chronic conditions: does prevalence pose a risk to medicare managed care? Managed Care Quarterly 2000; 8: 48-57.
25. Rohrer J, El-Urdaneta M, Vaughn T, Merchant JA. Physician visits in a farming-dependent county. Journal of Rural Health 1998; 14(4): 338-345.
26. Yesalis CE, Lemke JH, Wallace RB, Kahout FJ, Morris MC. Health status of the rural elderly according to farm work history: The Iowa 65+ Rural Health Study. Archives of Environmental Health 1985; 40: 245-253.
27. Nemet GF, Bailey AJ. Distance and health care utilization among the rural elderly. Social Science and Medicine 2000; 50: 1197-1208.
28. Wood E, Sallar A, Schechter M, Hogg R. Universal health care? Poor men more likely to die from "avoidable" causes in British Columbia. The evidence speaks, Monograph Series, (Monograph Number 1). No city: Centre for Health Evaluation and Outcome Sciences, 2003; 12. (Online) Available: http://www.cheos.ubc.ca (Accessed 4 Oct 2004).
29. British Columbia Provincial Health Officer. A report on the health of British Columbians: Provincial Health Officer's Annual Report 2001. Feature report: The health and well-being of Aboriginal people in British Columbia. Victoria, BC: Ministry of Health and Ministry Responsible for Seniors; 2002.
30. Gobrial M, Mekael H, Anderson N, Ayers D, HV Thommasen. Diabetic blood sugar control: an urban/rural comparison. British Columbia Medical Journal 2002; 44: 537-543.
31. Canadian Institute for Health Information. Aboriginal Peoples' Health in improving the health of Canadians. (Online) 2004. Available: http://www.cihi.ca (Accessed 4 Oct 2004).
32. Acheson S. Culture, contact, demography and health among the Aboriginal peoples of British Columbia. In: PH Stephenson, SJ Elliott, LT Foster, J Harris (Eds). A persistent spirit: towards understanding Aboriginal health in British Columbia. Canadian Western Geographical Series, Volume 31. Victoria: University of Victoria, Victoria. Western Geographical Press, 1995; 1-42.
33. Young TK, Szathmary EJE, Evers S, Wheatley B. Geographical distribution of diabetes among the native population of Canada: A national survey. Social Science and Medicine 1990; 31: 129-139.
34. Johnson S, Martin D, Sarin C. Diabetes mellitus in the First Nations population of British Columbia, Canada: Part 3. Prevalence of diagnosed cases. International Journal of Circumpolar Health 2002; 61: 260-264.
35. Martin JD, Yidegiligne HM. Diabetes mellitus in the First Nations population of British Columbia, Canada. International Journal of Circumpolar Health 1998; 57: 335-339.
36. Thommasen HV, Newbery P, Watt WD. Medical history of Central Coast of British Columbia. British Columbia Medical Journal 1999; 41: 464-470.
37. BC Stats. P.E.O.P.L.E. 27, BC Ministry of Management Services. PO Box 9410, Stn Prov Govt Victoria, BC; 2003.
38. British Columbia Vital Statistics Agency. 2001 British Columbia census. Victoria, BC: Government of British Columbia, 2003.
39. Kennedy DID, Bouchard RT. Bella Coola Indians. In: W. Suttles (Ed.) Handbook of North American Indians. Washington: Smithsonian Institute, 1990; 323-339.
40. McIlwraith TF. The Bella Coola Indians. Toronto: University of Toronto Press, 1992.
41. Thommasen HV. Prehistoric Medicine on BC's Central Coast. British Columbia Medical Journal 1999; 41: 343-346.
42. Patenaude J, Tildesley H, McArthur A, Thommasen A, Thommasen H. Prevalence of diabetes mellitus among Aboriginal and other people living in a rural and remote community - the Bella Coola Valley. Journal of Public Health 2004; (in press).
43. Smylie J. Aboriginal Health Issues Committee. A Guide for health professionals working with Aboriginal peoples: health issues affecting Aboriginal peoples. Journal SOGC 2001; 100: 54-68.
44. Macaulay AC, Gibson N, Freeman W et al. Participatory research maximizes community and lay involvement. BMJ 1999; 319: 774-778.
45. Cave AJ, Ramsden VR. Hypothesis: the research page. Participatory action research. Canadian Family Physician 2002; 48: 1671-1672.
46. Michalos AC, Thommasen HV, Anderson N, Zumbo B. Determinants of health and the quality of life in the Bella Coola Valley. Social Indicators Research 2004; (in press).
47. Meltzer S, Leiter L, Daneman D et al. 1998 clinical practice guidelines for the management of diabetes in Canada. Canadian Medical Association Journal 1998; 159(8): S1-S29.
48. Harvey G. EXCEL for Dummies, 2nd edn. Foster City, CA: IDG Books, 1994.
49. Snedecor GW, Cochran WG. Statistical Methods, 7th edn. Ames, Iowa: Iowa State University Press, 1980.
50. Thommasen HV, Thommasen C, Martiquet P, Jin A. Causes of death among status Indian and other people living in the Bella Coola Valley Local Health Area (1987-2001). British Columbia Medical Journal 2004; 46: 179-187.
51. Anon. Tobacco Use in BC 1997. A report on a survey done by the Heart and Stroke Foundation of BC and Yukon. (Online) 1997. Available: http:www.healthplanning.gov.bc.ca/tobacrs (Accessed 4 Oct 2004).
52. Statistics Canada. Canadian Community Health Survey (2000/2001). (Online) 2001. Available: http://www.statcan.ca/english/freepub/82-221-XIE/00503/tables/html/2115.htm (Accessed 4 Oct 2004).
53. Barr SI, Kuhnlein HV. HDL and total serum cholesterol levels in a group of British Columbia Native Indians. Nutrition Research 1985; 5: 827-837.
54. Kuhnlein HV, Chan AC, Thompson JN, Nakai S. Ooligan grease: A nutritious fat used by native people of coastal British Columbia. Journal of Ethnobiology 1982; 2: 154-161.
55. Kuhnlein HV. Traditional and contemporary Nuxalk food. Nutrition Research 1984; 4: 789-809.
56. Kuhnlein HV. Factors influencing use of traditional foods among the Nuxalk people. Journal of the Canadian Dietetic Association 1989; 50: 102-106.
57. Holub BJ. Dietary fish oils containing eicosapentaenoic acid and the prevention of atherosclerosis and thrombosis. Canada Medical Association Journal 1988; 139: 377-381.
58. Fauci AS, Braunwald E, Isselbacher KJ et al. Harrison's Principles of Internal Medicine, 14th edn. Toronto: McGraw-Hill, 1998.
59. MacLeod M, Browne AJ, Leipert B. Issues for nurses in rural and remote Canada. Australian Journal of Rural Health 1998; 6: 72-78.
60. Self B, Birmingham CL, Elliott R, Zhan W, Thommasen HV. Prevalence of overweight adults living in a rural and remote community - the Bella Coola Valley 2004. European Journal of Obesity and Eating Disorders 2004; (in press).
Abstract
Introduction: Objectives: (1) To identify which medical disorders are significantly associated with being a diabetic in the setting of an isolated, rural community; and (2) to determine if there are differences between Aboriginal and non-Aboriginal diabetics.
Methods: Design: population based retrospective chart review. Study population: people living in the Bella Coola Valley, Canada, and having a chart at the Bella Coola Medical Clinic as at September 2001. Main outcome measures: known diabetes related co-morbidity (retinopathy, nephropathy, coronary artery disease, peripheral vascular disease, neuropathy).
Results: There were 126 adult (>18 years old) diabetics living in the Bella Coola Valley. Prevalence rates for history of alcohol issues, retinopathy, coronary artery disease, cerebrovascular disease, peripheral vascular disease, peripheral neuropathy, hypertension, hypercholesterolemia, and nephropathy were 44%, 14%, 19%, 8%, 7%, 10%, 54%, 47%, and 7% respectively. For the 1597 non-diabetics living in the Bella Coola Valley, respective prevalence rates for these same co-morbidities were 20%, 0.3%, 2%, 1.5%, 1%, 1%, 10%, 6%, and 0.6%. The study did not demonstrate that Aboriginal people living in the Bella Coola Valley have an increased prevalence of diabetes associated co-morbidities over and above that found in the non-Aboriginal diabetic population. This was despite the fact the smoking rate was higher in the Aboriginal population.
Conclusion: The development of diabetes in both Aboriginal and non-Aboriginal people living in the Bella Coola Valley was clearly associated with the presence of multiple co-morbidities, including hypertension, hypercholesterolemia, coronary artery disease, cerebrovascular disease, and neuropathy. Rates of diabetes associated co-morbidities were similar for both Aboriginal and non-Aboriginal diabetic populations. The authors speculate that a diet rich in fish oils (omega-3 fatty acids) accounted for the lower than expected rates of cardiovascular disease among this Aboriginal population.
Key words: Aboriginal research, diabetes mellitus, diabetes related disease and morbidity, rural community.





