full article:
Introduction
Social disparities in cancer present an interesting challenge. This research area has received much attention, but there are still some basic misconceptions, even in the case of the phrase 'cancer disparities'1. Summarized results of this research area suggest that social disparities in cancer remain serious and persistent, despite major advances in the extent, determinants, treatment and prevention of cancer2. In an attempt to further disentangle cancer development mechanisms, three large factor groups were identified: (i) cumulative economic deprivation; (ii) exposure, susceptibility and resistance across the life course; and (iii) gene expression, not just gene frequency1.
Screening is an important tool for early cancer detection and consecutive mortality reduction. Various countries have differing guidelines, and varying policy implications for cancer screening programs (in both screening frequency and the respondent's age). Despite these efforts, a number of studies have shown that socio-economic determinants have an important role in actual screening uptake. An association between higher socioeconomic status and more frequent screening utilization has been described in the cases of breast3,4, prostate5-7 and colon cancer screening4,8. A study from California suggests that a decrease in colon and rectal cancer incidence may be related to wide-spread screening, especially among non-Hispanic white men and women who are considered to be the highest socio-economic group9. Additionally, it seems that rural origin has an important effect on the breast cancer screening uptake3, even after adjustment to socioeconomic factors.
Cancer is ranked the second commonest cause of death in Croatia, with steady and constant increase in the overall incidence10. The commonest male cancer sites in 2003 were trachea, bronchus and lung (21%), colon and rectum (14%), prostate (13%), and stomach (7%). Top cancer sites among women were breast (25%), colon and rectum (13%), trachea, bronchus and lung (6%) and uterine body (5%)10. At that time there was no national cancer screening program11.
The aim of this study was to investigate social disparities in breast, colon and prostate cancer screening in a sample of the adult Croatian population, with a special interest in the rural versus urban respondent's origin.
Methods
Setting
We investigated breast, colon and prostate cancer screening in a sample of the adult Croatian population. Data from the Croatian Adult Health Survey 2003 were used.
Croatian Adult Health Survey
The Croatian Adult Health Survey (CAHS) was designed to be a periodic survey of the Croatian population, aiming to provide surveillance of various risk factors12,13. The CAHS sample was defined on the basis of the 2001 Census of Population, in cooperation with the Central Bureau of Statistics of the Republic of Croatia. The survey targeted persons aged 18 years or older who were living in private dwellings in Croatia (those in non-conventional dwellings, clientele of institutions, full-time members of the Croatian Armed Forces and residents of certain remote and island regions were excluded from the survey). Sample size was targeted at 10 766 households, which was stratified to six regions of Croatia, with coverage estimated as approximately 98% of the Croatian adult population. Public health nurses surveyed respondents in their homes during May and July 200313. One adult inhabitant was randomly chosen from each household. The final collected sample consisted of 9070 respondents, with a response rate of 84.2%. Finally, a weighting scheme was applied to the dataset, further increasing the representativeness of the CAHS sample and enabling the projecting of results to the entire Croatian population12. The Ethical Board of the Medical School, University of Zagreb approved the study.
Measurements
Questions on breast, prostate and colon cancer screening utilization during the year preceding the survey were used. Responses were coded as binary outcomes. Breast screening utilization was investigated in a sub-sample of women aged over 40 years, while prostate and colon cancer screening were investigated in a sub-sample of respondents aged over 50 years. Questions were broad because clear differentiation of screening method was not possible (eg whether colon screening was digito-rectal examination, sigmoidoscopy, colonoscopy or a faecal occult blood test; or if prostate screening was digito-rectal examination or using PSA testing).
Factors used as socioeconomic determinants were: education level (classified in four groups; without completed primary school, completed primary school, completed secondary school, and university degree); objective household income (classified as the ordinal measure of four classes, expressed in the Croatian currency, Kuna); and occupation (binary; white- or blue-collar occupations). Rural versus urban origin was assessed by the respondent's permanent address, according to the rural versus urban location classification from the Central Bureau of Statistics and the Governmental classification of the rural and urban settlements. Additionally, subjective healthcare access estimates were calculated. Respondents had the opportunity to score the accessibility of their general practitioner, polyclinic and hospital from 1 to 3 (1 = no problems; 2 = moderate; and 3 = a substantial problem in healthcare access). Respondents who scored at least 6 points when all three variables were summed were considered to have a healthcare access problem. Health insurance was not included as an independent variable because there is almost complete obligatory health insurance coverage in Croatia.
Statistical analysis
Analysis was performed in SAS 8.0.2 (SAS; Carry, NC, USA), with bootstrapping variance estimation performed by the Bootvare_sas. v2012. All results were presented as weighted estimates for the entire Croatian population. Coefficient of variation (CV) was used as a variation indicator for the weighted screening estimates. The CV values of less than 16.6 were considered optimal; those between 16.6 and 33.3 were considered to reflect a greater extent of variation; while estimates over 33.3 were considered to reflect too much variation (consequently, these values were not considered reliable in the interpretation of results). Binary logistic regression was used in multivariable analysis, without interaction items. Bootstrap variance estimation was also used in the regression models. Screening was used as the dependent variable, with a number of other predictor variables in the models. Predictor variables included: age, education class, occupation, access to health care, rural versus urban origin, and household income estimate. All models were gender specific. Statistical significance was set at p<0.05.
Results
Analysis of the Croatian Adult Health Survey indicated that 22.5% of women (95% CI 21.3-23.8) aged over 40 years reported having a breast cancer screening during a year preceding the survey. A total of 13.7% of men (95% CI 11.4-15.9) aged over 50 years reported having a prostate screening. Fewer respondents reported having a colon screening during a year preceding the survey: 4.5% of women (95% CI 3.6-5.4) and 6.1% of men (95%CI 4.5-7.6).
Peak onset for breast cancer screening was reported in the 50-59 years age group, with a significant reduction in screening uptake in the oldest age groups (indicated by the non-overlap of the confidence intervals; Table 1). More frequent breast cancer screening uptake was reported by women in white-collar occupations and those from urban areas (Table 1). Similar results were recorded in prostate cancer screening in men, with less clear differences (Table 1). The oldest age group reported prostate screening most commonly, although a higher coefficient of variation was recorded for this estimate (Table 1). All differences in colon screening in both genders were less marked, sometimes without clear differences. The 60-69 years age group in both genders reported peak onset for colon screening (Table 1). Screening utilization was systematically less commonly reported by respondents with a rural origin, compared with those of urban origin (Table 1).
Table 1: Social disparities in utilization of screening services during the year preceding the survey, from the Croatian Adult Health Survey 2003 sample. Numbers are given as percentages and 95% confidence intervals

A multivariable model of the self-reported breast cancer screening indicated that most of the investigated variables were significantly associated with screening uptake, except for lower education and occupation classes (Table 2). The respondent's age, two classes of educational level, occupation and access to health care were significantly associated with prostate screening (Table 2). Access to health care was the only significant independent variable associated with colon screening in men (Table 3). Women coming from households with the highest incomes, and those who reported having no problems in access to health care most often reported having a colon screening within the last year (Table 3).
Table 2: Logistic regression models of breast and prostate screenings during the year preceding the survey, from the Croatian Adult Health Survey 2003 sample
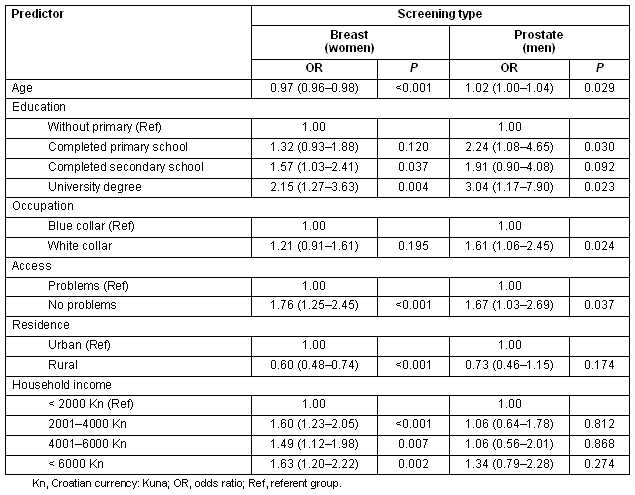
Table 3: Logistic regression models of colon screenings during the year preceding the survey, from the Croatian Adult Health Survey 2003 sample

Discussion
The results of this study indicate infrequent utilization of cancer related screening in the adult Croatian population. The effects of post-war health system transition or the lack of national screening programs may have contributed to the current situation. There are, however, some preventive local actions (such as 'breast cancer awareness day'), or non-systematic screening efforts (such as the 'mobile mammography project', which aims to reach population fractions who have difficulty in accessing health care). In this situation, the effects of socio-economic determinants and a respondent's urban origin could hypothetically be even more pronounced than in other countries that have implemented systematic screening programs, because people have more individual responsibility in screening services utilization (combined with recommendations from their physicians).
Social disparities in breast cancer screening have been extensively described, usually reporting the worst indices among women from lower socioeconomic classes3. These women are less likely to respond to a screening invitation14,15, and they are at an increased risk of late-stage breast cancer diagnosis16. Interestingly, disparities in breast cancer screening remained even in a setting with continuity of health care17. Rural origin is a factor that has been associated with lesser probabilities of breast screening uptake3, although this finding does not seem to be universal18. The results of another study suggest that rural origin is not, of itself, a crucial negative breast cancer screening predictor, but an element of a much finer interplay of various factors19. The results of the present study contradict such a finding, because a lesser incidence of breast screening in rural women remained even after controlling for the most obvious confounding factors, general lower socio-economic status, older age of rural women, and difficulties in accessing the healthcare facilities.
Access to health care was a significant predictor of breast cancer screening in the present study, supporting some previous findings20. It has been implied that this is one of the most important factors for breast screening in limited-resource settings21. However, other studies suggest that, even in settings with the same access to mammography, women from lower socioeconomic classes were less likely to use this screening22. Overall findings from the present study supported clear socioeconomic differences in self-reported breast cancer screening uptake, suggesting that women from higher socioeconomic classes in Croatia are most likely to receive opportunistic breast screening.
In contrast to breast cancer screening, the overall effectiveness of prostate screening is far less convincing. There is still no consensus as to whether prostate screening is effective23, with some studies suggesting that screening for prostate cancer cannot be justified in low-risk populations24. Nevertheless, more frequent screening utilization among men of higher socioeconomic status has been implied in a number of studies5,6, even suggesting that when ethnic differences were diluted the socioeconomic differences persisted25. The current study also supports more frequent screening utilization among men from higher socioeconomic classes, but with less clear differences than those for breast cancer screening in women. It is worth noting that respondents of rural origin had lower odds ratio of prostate screening in the multivariable analysis, but that these differences were not statistically significant.
Access to health care was the only significant predictor of colon cancer screening in men. This finding suggests that either a model did not provide a good data fit (due to smaller sample sizes and, consequently, large variation), or that there is a true lack of socioeconomic disparity in colon cancer screening among men. Women with problems in healthcare access reported a more than three times less probability of receiving a colon cancer screening, suggesting that access to health care is the single most important predictor of the colon screening in both genders. In contrast to other screening methods, colon cancer screening effectiveness can be assessed by investigation of the cancer site. A shift from a predominance of a rectal location to more frequent right-sided colonic cancer has been associated with increased screening rates26. An investigation of the colon cancer sites from the National Cancer Registry of Croatia could provide the basis for evidence-based screening program evaluation, once a national screening program is developed and implemented.
Lack of national screening programs, and cancer mortality rates higher that in the European Union or European region prompted the creation of the 'National Cancer Prevention and Screening Programme Proposal'27. The Ministry of Health and Social Welfare launched a national screening program in 2006, which introduced regular mammography for women aged 50-69 years. Commencing in 2007, the program will introduce regular colon occult-blood screening for people over 50 years27.
The shortcomings of this study include the use of self-reported survey data, and the use of broad and unspecified questions. The use of targeted questions (on types of screening) supplemented with medical records would produce more precise estimates, and reduce the possibility of reporting diagnostic and therapeutic examinations as screening. There is an additional problem in colon cancer screening models, because some studies suggest that in systems with universal access to health care, approximately two-thirds of colonoscopies were performed due to known symptoms, while only one-third were performed as screening28.
Conclusions
This study reports an unsatisfactory cancer screening uptake among the adult population of Croatia. While we may speculate whether this situation occurred as a consequence of the war or health system transition, the increasing trend in cancer incidence and mortality continues. People with higher socioeconomic status more often reported breast and prostate screening; however, no association of socioeconomic status with colon cancer screening was detected. Rural origin was negatively associated with breast screening uptake, while the results from the other screening sites were less convincing. Overall results suggest that ensuring easier access to screening could increase the frequency of screening services utilization, with a final goal of cancer burden reduction.
Acknowledgement
Sources of funding: The Ministry of Science, Education and Sport of the Republic of Croatia (Regionalism of cardiovascular behavioural risk factors - model of intervention; No: 108-1080135-0264).
References
1. Krieger N. Defining and investigating social disparities in cancer: critical issues? Cancer Control 2005; 16: 5-14.
2. Krieger N, Emmons KM, White KB. Cancer disparities: developing a multidisciplinary research agenda - preface. Cancer Control 2005; 16: 1-3.
3. Bigby J, Holmes MD. Disparities across the breast cancer continuum. Cancer Control 2005; 16: 35-44.
4. Koh HK, Judge CM, Ferrer B, Gershman ST. Using public health data systems to understand and eliminate cancer disparities. Cancer Control 2005; 16: 15-26.
5. Gilligan T. Social disparities and prostate cancer: mapping the gaps in our knowledge. Cancer Control2005; 16: 45-53.
6. Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Preventive Medicine 2004; 38: 732-744.
7. Finney Rutten LJ, Meissner HI, Breen N, Vernon SW, Rimer BK. Factors associated with men's use of prostate-specific antigen screening: evidence from Health Information National Trends Survey. Preventive Medicine 2005; 40: 461-468.
8. Palmer RC, Scneider EC. Social disparities across the continuum of colorectal cancer: a systematic review. Cancer Control 2005; 16: 55-61.
9. Cress RD, Morris CR, Wolfe BM. Cancer of the colon and rectum in California: trends in incidence by race/ethnicity, stage, and subsite. Preventive Medicine 2000; 31: 447-453.
10. Strnad M. Cancer Incidence in Croatia 2003, Bulletin no. 28. Zagreb: Croatian National Institute of Public Health, 2005.
11. Sansom C. Provision of cancer care in Eastern Europe. The Lancet Oncology 2002; 3: 203-205.
12. Béland Y, Bailie L, Page J. Statistics Canada, Croatian Ministry of Health and Central Bureau of Statistics: a joint effort in implementing the 2003 Croatian Adult Health Survey. Proceedings of the American Statistical Association Meeting, Survey Research Methods. Toronto: American Statistical Association, 2004.
13. Vuletic S, Kern J. Hrvatska zdravstvena anketa 2003. [Croatian Adult Health Survey 2003]. Hrvatski casopis za javno zdravstvo 1: 7. (Online) 2005. Available: http://www.hcjz.hr/clanak.php?id=12389 (Accessed 11 May 2006). [In Croatian]
14. Kaffashian F, Godward S, Davies T, Solomon L, McCann J, Duffy SW. Socioeconomic effects on breast cancer survival: proportion attributable to stage and morphology. Breast Cancer Journal 2003; 89: 1693-1696.
15. Baum M. Breast cancer screening comes full circle. Journal of the National Cancer Institute 2004; 96: 1490-1491.
16. Arndt V, Sturmer T, Stegmaier C, Ziegler H, Dhom G, Brenner H. Socio-demographic factors, health behavior and late-stage diagnosis of breast cancer in Germany: a population-based study. Journal of Clinical Epidemiology 2001; 54: 719-727.
17. Menec VH, Sirski M, Attawar D. Does continuity of care matter in a universally insured population? Health Services Research 2005; 40: 389-400.
18. Fukuda Y, Nakamura K, Takano T. Reduced likelihood of cancer screening among women in urban areas and with low socio-economic status: a multilevel analysis in Japan. Public Health 2005; 119: 875-884.
19. Cummings DM, Whetstone LM, Earp JA, Mayne L. Disparities in mammography screening in rural areas: analysis of county differences in North Carolina. Journal of Rural Health 2002; 18: 77-83.
20. Aldridge ML, Daniels JL, Jukic AM. Mammograms and healthcare access among US Hispanic and non-Hispanic women 40 years and older. Family Community Health 2006; 29: 80-88.
21. Smith RA, Caleffi M, Albert US, Chen TH, Duffy SW, Franceschi D, Nystrom L. Global Summit Early Detection and Access to Care panel. Breast cancer in limited-resource countries: early detection and access to care. Breast Journal 2006; 12(Suppl 1): 16-26.
22. Royak-Schaler R, Chen S, Zang E, Vivacqua RJ, Bynoe M. Does access to screening through health maintenance organization membership translate into improved breast cancer outcomes for African American patients? Journal of the American Medical Womens Associatiion 2003; 58: 154-156.
23. Martin RM, Smith GD, Donovan J. Does current evidence justify prostate cancer screening in Europe? National Clinics of Practical Oncology 2005; 2: 538-539.
24. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003; 361: 1122-1128.
25. . Fowke JH, Schlundt D, Signorello LB, Ukoli FAM, Blot WJ. Prostate cancer screening between low-income African-American and Caucasian men. Urology and Oncology 2005; 23: 333-340.
26. Sarli L, Michiara M, Sgargi P, Iusco D, De Lisi V, Leonardi F, Bella MA, Sgobba G, Roncoroni L. The changing distribution and survival of colorectal carcinoma: an epidemiological study in an area of northern Italy. European Journal of Gastroenterology and Hepatology 2005; 17: 567-572.
27. Samija M, Strnad M, Ebling Z, Kovacic L, Znaor A. National Cancer Prevention and Screening Programme proposal. Zagreb: Croatian Ministry of Health and Social Welfare, 2006. [In Croatian]
28. Bressler B, Lo C, Amar J, Whittaker S, Chaun H, Halparin L, Enns R. Prospective evaluation of screening colonoscopy: who is being screened? Gastrointestinal Endoscopy 2004; 60: 921-926.





