Globally, Australia and New Zealand, North America, and Northern and Western Europe have the highest incidences of colorectal cancer (CRC)1-3. In Australia, CRC is the second most commonly diagnosed cancer and cause of death from malignant diseases4, with the CRC crude rate increasing and predicted to rise by approximately 30% between 2002 and 20115.
A number of randomised controlled trials have demonstrated the effectiveness of CRC screening for reducing CRC incidence and mortality6-8. However, the impact of screening on CRC incidence and mortality has been limited by a number of factors, including the accuracy of screening technology9, the willingness of eligible populations to participate10, access to CRC screening6,7 and primary healthcare practitioners11, geographical location12, Indigenous status13-15, and a range of social, demographic, and economic factors16,17.
The Australian CRC population-based screening program, the National Bowel Cancer Screening Program (NBCSP) was first implemented in South Australia (SA) in February 2007. The first phase of the NBCSP (timeframe of this study) offered free CRC screening in the form of an immunochemical faecal occult blood test (FOBT) test for people turning 55 and 65 years of age between 1 May 2006 and 30 June 2008. A pre-invitation letter and subsequent invitation package including FOBT test kit are posted to eligible participants by Medicare Australia. Invitees are requested to mail their FOBT sample kit to a central pathology service for analysis.
Although the Australian NBCSP provides universal access to the screening program, early findings from the analysis of the NBCSP at the national level demonstrate that there are differences in participation rates among population sub-groups and geographical locations3,17-19.
Participation in preventive programs including cancer screening programs has been shown to be associated with geographical location. People living in rural and remote areas are less likely than metropolitan residents to access preventive health services20,21, including cancer screening services22-24. Overall, poorer health outcomes increase with geographic isolation, and rural and remote residents are less likely to be diagnosed with localised disease, and hence have higher cancer-specific mortality rates25.
Rural and remote residents are less likely to participate in the CRC screening program compared with their urban counterparts26. Lower rates of screening participation in rural areas have been attributed to lower socioeconomic status, lack of access to healthcare services, low health literacy, exclusive reliance on Medicare coverage, and social isolation25,27. Inadequate patient-provider communication about CRC screening26 and distance to travel to a GP28 have also been found to be barriers to CRC screening in rural primary care.
This article explores participation in the NBCSP for people residing in geographically rural and remote areas of SA. The focus is to identify population groups within these geographical areas who may benefit from targeted programs to increase participation rates in the NBCSP.
Sample
The target population included NBCSP invitees in SA who turned 55 and 65 years between 2007 and 2008 and were invited by Medicare Australia to complete the FOBT testing kit which had been mailed to them. The NBCSP participants are defined as invitees who undertook the FOBT screening and returned NBCSP Participant Information Form to a central pathology service for analysis, and had a positive or negative test result.
Sampling frame
Data for this study were based on de-identified, South Australian Medicare Australia extract between February 2007 and July 2008 (phase 1 of the program). The dataset for the NBCSP invitees (total number = 92 279) included data on age, sex and postcode; for NBCSP participants it also included data on Indigenous status and language spoken at home. For the purpose of this study, the 17 497 South Australians who participated in the pilot phases of the NBCSP were removed from the dataset because their exposure to NBCSP may have a confounding effect on subsequent NBCSP participation. Therefore, the final dataset for analysis included 74 782 South Australians who had been invited to undertake screening for the first time by the NBCSP.
Ethics approval
Ethics committee approval was granted by the Ethics Committee of the Commonwealth Department for Health and Ageing, and by the Social and Behavioural Research Ethics Committee of Flinders University.
Data analysis
Medicare data were analysed using the Statistical Package for the Social Sciences (SPSS) v17.0 (www.spss.com). Initial analyses were undertaken using χ2 testing and univariate regression analyses in order to describe the associations between participation in the NBCSP and the socio-demographic variables: age, sex, postcode, Indigenous status and language spoken at home. All univariate variables found to be associated with the dependent variable, NBCSP participation, at p<0.25 level29 were entered as independent variables into a logistic regression analysis (block-enter method). Indigenous status and language spoken at home were not included in the regression model because the data were not available for NBCSP invitees. The final multiple regression model was checked for collinearity and all variables included at the initial step remained statistically significant.
Postcodes were re-coded for the analysis in the following way. First, postcodes were converted into a measure of remoteness, using the Accessibility/Remoteness Index of Australia (ARIA)30. This is an index of the proximity of postcodes to service centres, or conversely of remoteness of postcodes. The ARIA has both a 5 point and a 3 point scale. The 3 point scale used in this study includes the following categories: (i) ARIA 0-1.84, indicating highly accessible or metropolitan locations; (ii) ARIA >1.84-5.80 indicating accessible and moderately accessible or rural areas; and (iii) ARIA >5.80-12.0, indicating remote and very remote areas. Second, each postcode was coded according to the Socio-Economic Indexes for Areas (SEIFA)-Index of Relative Socio-Economic Disadvantage (IRSD)31, a composite measure based on selected census variables such as income, educational attainment and employment status. The SEIFA-IRSD scores for each postcode were then grouped into quintiles, where the highest quintile consists of the 20% of postcodes with the highest SEIFA-IRSD scores and represents the least disadvantaged areas; and the lowest quintile consists of the 20% of postcodes with the lowest scores and represents the most disadvantaged areas. Mapping and analysis of the NBCSP data was performed using ArcGIS (http://www.esri.com/software/arcgis/index.html), MapInfo (http://slp.pbinsight.com/info/mipro-sem-au) and MS Access and Excel.
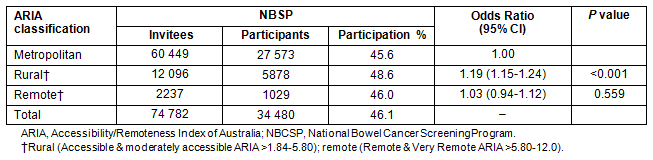
The profile of NBCSP participants in SA indicates that participation varied by place of residence as defined by ARIA classification, age, sex , Indigenous status, language spoken at home and social disadvantage as measured by SEIFA-IRSD (Table 1). Table 2 demonstrates rates of participation in the NBCSP by geographical location.
Table 1: Profile of South Australian National Bowel Cancer Screening Program participants by Accessibility/Remoteness Index of Australia
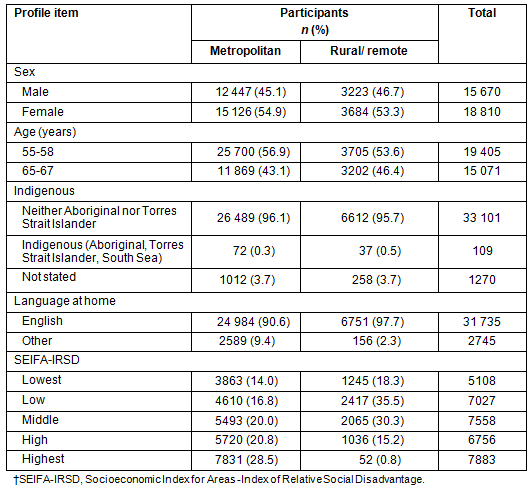
Table 2: South Australian participation rates by Accessibility/Remoteness Index of Australia
Geographical postcode: Of the 74 782 SA invitees, 34 480 participated in the NBCSP, an overall participation rate of 46.1% (Table 2). Of those participating, 6907 (20%) were from areas classified as rural or remote. Participation rates by place of residence and geographical accessibility as classified by ARIA, were found to be significantly different between SA participants residing in metropolitan locations (45.6%) and those residing in rural and remote areas combined (48.2%). However, compared with metropolitan areas, while NBCSP participation rates are marginally similar in remote areas, they are statistically significantly higher in rural areas (Table 2).
The map of NBCSP participation rates in rural and remote SA (Fig1) reveals a pattern of high participation in screening in Eastern and Southern SA, with screening rates ranging between 60% and 100% in some postcodes. As can be seen, there was insufficient data to calculate participation rates in large sections of the state, particularly in the Far North subdivision of SA.
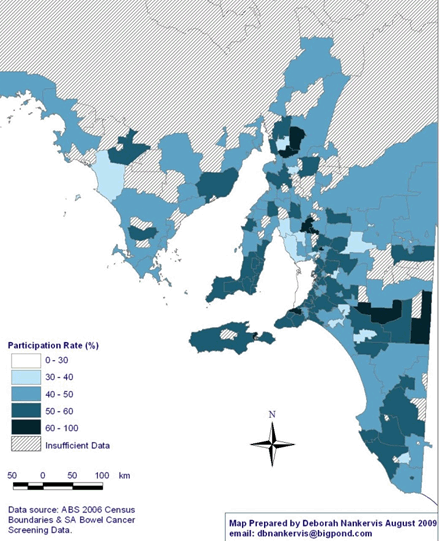
Figure 1: National Bowel Cancer Screening Program participation rates (% ranges) in rural and remote South Australia by postcode.
Age: The NBCSP participation rates vary significantly according to participant profile (Tables 3 and 4). Comparing NBCSP participation by age indicates that rural and remote SA invitees who were aged 55 years were significantly less likely to respond to screening invitation by undertaking FOBT than those aged 65 years, taking into account other participants' variables. There were higher participation rates in the southern and eastern areas of SA for participants aged 65 years (Fig2). Overall, screening rates of 60-100% were three times more in the 65 years age group than in the 55 years age group. Similar patterns of geographical disparity exist, with Far North SA having lower rates of participation in both age groups.
Table 3: Univariate odds ratios for National Bowel Cancer Screening Program (National Phase 1) participation rates in rural and remote South Australia, by Accessibility/Remoteness Index of Australia and participant profile
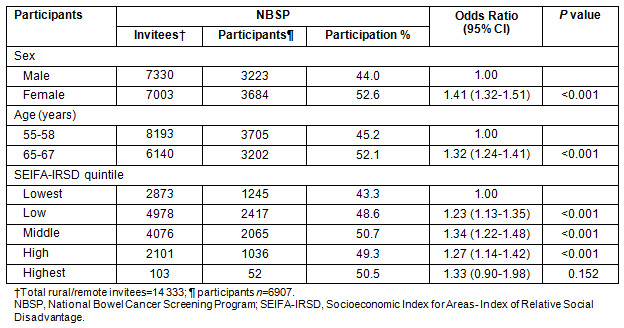
Table 4: Multivariate odds ratios for participation in National Phase 1 in rural and remote areas29
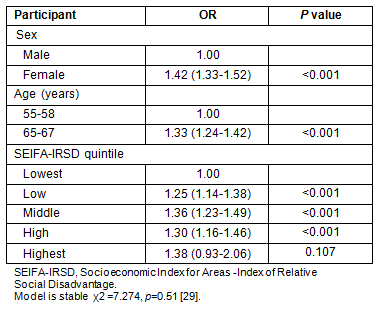
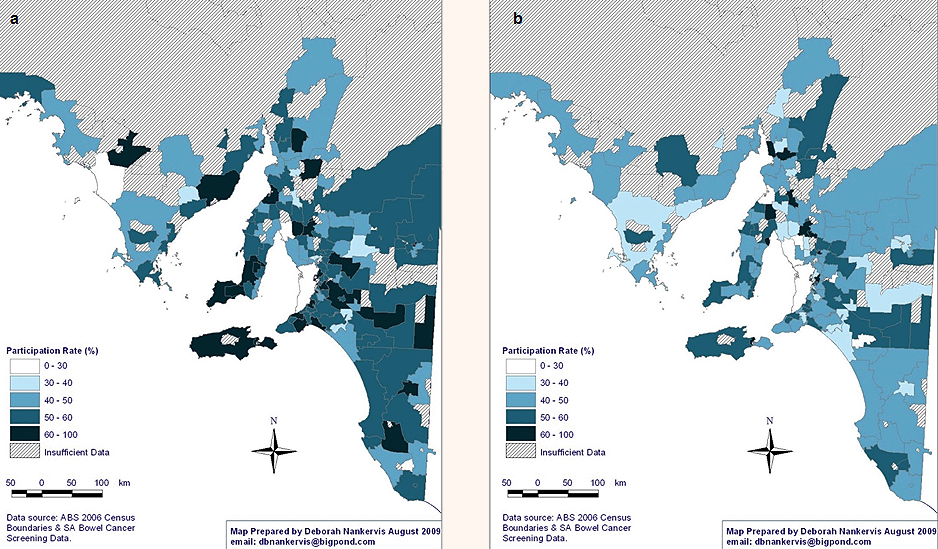
Figure 2: National Bowel Cancer Screening Program participation rates in rural and remote South Australia at age: (a) 55 years, and (b) 65 years.
Sex: Male participation in NBCSP was significantly lower than among females. There were large differences in participation according to sex (Fig3), with female participation rates of 60-100% being significantly higher than for men, in most areas.
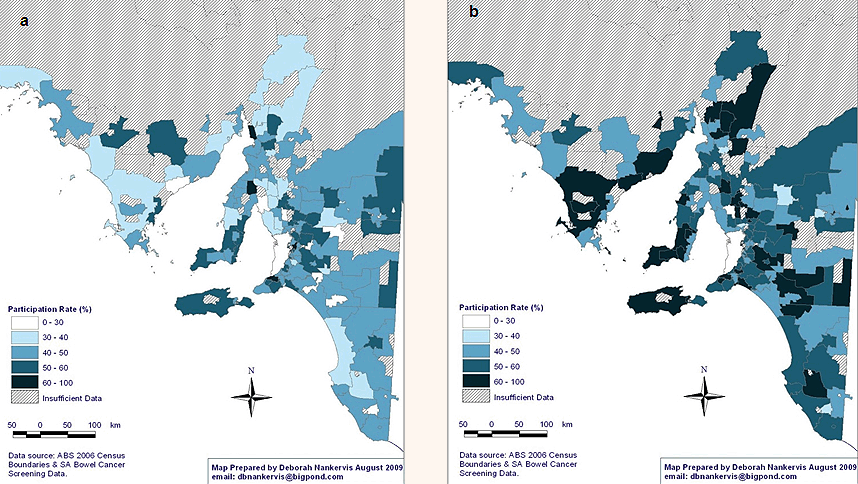
Figure 3: National Bowel Cancer Screening Program participation rates in rural and remote South Australia according to sex: (a) male, and (b) female.
SEIFA-IRSD: Participation rates by SEIFA-IRSD were significantly different. Tables 3 and 4 reveal a gradient in participation where the lowest two quintiles, which represent greater social disadvantage, have significantly lower participation rates than the middle and highest quintiles.
Indigenous race: Self-reported Aboriginal and Torres Strait Islander status (in this article referred to as Indigenous) was available only for participants who completed the FOBT, with the Indigenous status of invitees who failed to return their FOBT unknown. The total proportion of the Indigenous participants from rural and remote areas was 0.10% (0.06-0.15). Given that Indigenous Australians comprise approximately 1.7% of the South Australian population32 and, of these, 0.62% are 55 years of age, and 0.47% are 65 years, 7.0% live in remote areas (0.2% of total population), and 2.9% within rural areas (0.4% of total population)33, and as this proportion is not within the confidence interval of the sample, the proportion of Indigenous people who participated in the NBCSP was statistically significantly lower than was expected. However, given the insufficient details about the Indigenous status of invitees, it was not possible to calculate an overall participation rate. Significantly, the areas showing unknown participation rates (Fig1) have high Indigenous populations.
Languages spoken at home: In rural and remote areas 2.3% of participants reported speaking a language other than English at home. Australian Bureau of Statistics 2006 statistics show this population for Northern SA as 7.1%, and Murraylands 6.2%34. Given the ABS statistics, this participation is lower than could be expected.
Limitation of the data
It was not possible to ascertain if South Australians who had been invited to undertake screening for the first time by the NBCSP had previously been offered, or participated in, CRC screening other than the NBCSP.
Discussion
Overall, the analyses revealed lower NBCSP participation rates for people from metropolitan and remote areas compared with those from rural areas. Within these areas, lower participation rates were found for men compared with women, those 55 years compared with 65 year olds, and socio-economically disadvantaged groups compared with more affluent groups. Among invitees who participated in the NBCSP, comparison with the most recent Australian Census data indicated that South Australians who reported speaking a language other than English at home and those who reported an Indigenous background were under-represented. These differences in participation rates were consistent for CRC screening in the SA metropolitan state capital Adelaide12, and NBCSP national data35-37. These findings are also consistent with results from other cancer screening programs which suggest that inequitable patterns of participation may arise from a variety of factors including those associated with sex38,39 , age, socio-economic status40,41 and Indigenous status42,43. The finding of increasing participation in rural areas was inconsistent with much of the literature where evidence shows that non-attendance at screening was positively associated with living in a rural area23,24.
Significantly, the data suggests inequitable patterns of participation across geographical lines within rural and remote SA. Geographically, the pattern of high participation in CRC screening in Eastern and Southern SA, compared with the significantly large areas of low participation rates in Far North SA, are identified by the most recent ABS census34 as areas with large Indigenous and more socially disadvantaged populations.
While the national monitoring of the NBCSP44 is useful, it does not provide the necessary details at a state-based level (and lower levels of aggregation such as postcodes) required to inform service planning. Moreover, it does not highlight the specific geographical areas that might benefit from renewed attempts at targeted interventions designed to improve participation. The maps of NBCSP participation rates provided in this study (Figs1,2&3) reveal differences among South Australian postcodes in terms of rural and remote area participation rates, although these differences may have been accounted for in part by the above variables.
The greatest disparities in CRC screening in rural and remote SA were associated with socioeconomic disadvantage, sex, age and Indigenous status factors. Therefore, population-based screening programs that target these identified groups will offer rural and remote residents the same opportunity access to CRC screening.
The early detection of CRC is a major clinical and public health concern. The findings indicate that residing in rural and remote areas can affect uptake of cancer screening, with sex, age, Indigenous status and SEIFA-IRSD having a particularly negative impact on screening. Older rural and remote residents, men, Indigenous peoples and those living in Far North SA need specifically targeted preventive screening services. The special challenges that geographically isolated areas present highlight the need for more research in order to understand the reasons for disparities in screening participation and areas of insufficient data.
References
1. Max Parkin D, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA: A Cancer Journal for Clinicians 2005; 55: 74-108.
2. Westlake S, Cooper N. Cancer incidence and mortality: trends in the United Kingdom and constituent countries, 1993 to 2004. Health Statistics Quarterly 2088; 38: 33-46.
3. American Cancer Society. Colorectal Cancer Facts& Figures 2008-2010. Atlanta, GA: American Cancer Society, 2008.
4. Australian Institute of Health and Welfare, and Australasian Association of Cancer Registries. Cancer in Australia: an overview, 2008. Canberra, ACT: AIHW and AACR, 2008.
5. McDermid I. Cancer Incidence Projections Australia 2002 to 2011. Canberra, ACT: Australian Institute of Health and Welfare, and Australasian Association of Cancer Registries, The National Cancer Strategies Group, 2005.
6. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348: 1467-1471.
7. Hardcastle JD, Chamberlain JO, Robinsonk MH, Moss SM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348: 1472-1477.
8. Mandel J, Church T, Bond J. The effect of fecal occult-blood screening on the incidence of colorectal cancer. New England Journal of Medicine 2000;343: 1603-1607.
9. Whitlock E, Lin J, Liles E, Beil T, Fu R. Screening for Colorectal Cancer: A Targeted, Updated Systematic Review for the US Preventive Services Task Force. Annals of Internal Medicine 2008; 149: 638-658.
10. Ford J, Howerton M, Lai G, Gary T, Bolen S, Gibbons M et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008; 112: 228-242.
11. Hamilton W. Five misconceptions in cancer diagnosis. British Journal of General Practice 2009; 59: 441-447.
12. Javanparast S, Ward P, Cole S, Gill T, Ah Matt M, Aylward P et al. A cross-sectional analysis of participaion in National Bowel Cancer Screening Program in Adelaide by age, gender and geographical location of residence. Australasian Medical Journal 2010; 1: 141-146.
13. Condon JR, Armstrong BK, Barnes A, Cunningham J. Cancer in Indigenous Australians: a review. Cancer Causes & Control 2003; 14: 109-121.
14. Condon JR, Armstrong BK, Barnes T, Zhao Y. Cancer incidence and survival for indigenous Australians in the Northern Territory. Australian & New Zealand Journal of Public Health 2005; 29: 123-128.
15. Condon JR, Barnes T, Armstrong BK, Selva-Nayagam S, Elwood JM. Stage at diagnosis and cancer survival for Indigenous Australians in the Northern Territory. Medical Journal of Australia 2005; 182: 277-280.
16. Whynes DK, Frew EJ, Manghan CM, Scholefield JH, Hardcastle JD. Colorectal cancer, screening and survival: the influence of socio-economic deprivation. Public Health 2003; 117: 389-395.
17. Javanparast S, Ward P, Young G, Wilson C, Carter S, Mison Get al. How equitable are colorectal cancer screening programs which inlcude FOBTs? A review of qualitative and quantitative studies. Preventive Medicine 2010; 50: 165-172.
18. Weller D, Coleman D, Robertson R, Butler P, Melia J, Campbell C et al. The UK colorectal cancer screening pilot: results of the second round of screening in England. British Journal of Caner 2007; 97: 1601-1605.
19. Whynes D, Frew E, Manghan C, Scholefield J, Hardcastle J. Colorectal cancer, screening and survival: the influence of socio-economic deprivation Public Health 2003; 117: 389-395.
20. Casey M, Thiede-Call K, Klingner J. Are rural residents less likely to obtain recommended preventive healthcare services?. American Journal of Preventive Medicine 2001; 21: 182-188.
21. Larson S, Correa-de-Araujo R. Preventive health examination: a comparison along the rural-urban continum. Women's Health Issues 2006; 16: 80-88.
22. Borders TF, Warner RD, Sutkin G. Satisfaction with health care and cancer screening practices among women in a largely rural region of West Texas. Preventive Medicine 2003; 36: 652-658.
23. Eaker S, Adami H.-O, Sparen P. Reasons Women Do Not Attend Screening for Cervical Cancer: A Population-Based Study in Sweden. Preventive Medicine 2001; 32: 482-491.
24. Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women. Social Science and Medicine 2008; 66: 260-275.
25. Popa M, Extermann M. Survival in older adults with a history of cancer: Is rural residence a negative prognostic factor? Critical Reviews in Oncology/Hematology 2008; 68: S31-S31.
26. Greiner KA, Engelman KK, Hall MA, Ellerbeck EF. Barriers to colorectal cancer screening in rural primary care. Preventive Medicine 2004; 38: 269-275.
27. Smith KB, Humphreys JS, Wilson MGA. Addressing the health disadvantage of rural populations: How does epidemiological evidence inform rural health policies and research?. Australian Journal of Rural Health 2008; 16: 56-66.
28. Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel times to health care and survival from cancers in Northern England. European Journal of Cancer 2008; 44: 269-274.
29. Hosmer D, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons, 2000.
30. Australian Institute of Health and Welfare. Rural, regional and remote health: a guide to remoteness classifications. Canberra, ACT: AIHW, 2004.
31. Australian Bureau of Statistics. Census of Population and Housing: Socio-EconomicIndexes for Area's (SEIFA). Canberra, ACT: ABS, 2004.
32. Australian Bureau of Statistics. National Regional Profile: South Australia. Canberra, ACT: ABS, 2006.
33. Australian Bureau of Statistics. Experimental Estimates of Aboriginal and Torres Strait Islander Australians. Canberra, ACT: ABS, 2006.
34. Australian Institute of Health and Welfare. National Bowel Cancer Screening Program monitoring report 2008. Canberra, ACT: AIHW, 2008.
35. Australian Institute of Health and Welfare. National Bowel Cancer Screening Program monitoring report 2007. Canberra, ACT: AIHW, 2007.
36. Australian Institute of Health and Welfare. National Bowel Cancer Screening Program monitoring report 2009. Canberra, ACT: AIHW, 2009.
37. Friedemann-Sanchez G, Griffin JM, Partin MR. Gender differences in colorectal cancer screening barriers and information needs. Health Expectations 2007; 10: 148-160.
38. Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. Journal of Community Health 2000; 25: 263-278.
39. McCaffery K, Wardle J, Nadel M, Atkin W. Socioeconomic variation in participation in colorectal cancer screening. Journal of Medical Screening 2002; 9: 104-108.
40. Wardle J, McCaffery K, Nadel M, Atkin W. Socioeconomic differences in cancer screening participation: comparing cognitive and psychosocial explanations. Social Science and Medicine 2004; 59: 249-261.
41. Ward PR, Kelly B, Tucker G, Luke C. Theoretical and conceptual issues around equity in health care: application to cervical cancer screening in South Australia. Public Health Bulletin 2006; 5: 9-14.
42. Binns PL, Condon JR. Participation in cervical screening by Indigenous women in the Northern Territory: a longitudinal study. Medical Journal of Australia 2006; 185: 490-494.
43. Australian Bureau of Statistics. Australian Social Trends. Canberra, ACT: ABS, 2004.
44. Australian Institute of Health and Welfare, and Australian Government Department of Health and Aging. National Bowel Cancer Screening Program monitoring report 2008. Canberra, ACT: AIHW and AACR, 2008.



