Introduction
Osteoarthritis (OA) is a prevalent chronic musculoskeletal (MSK) disease that causes pain, joint stiffness and muscle weakness, which impacts physical function and quality of life1-5. According to the Global Burden of Disease Study, MSK diseases are the second greatest cause of disability worldwide, with the greatest increase of disability over the past 20 years6. Projected estimates of OA are affiliated with an aging population and the obesity epidemic7-9. In Canada, the prevalence of OA is projected to increase from 13.6%10 to 25% in the general population by 2040, and up to 30% of the workforce will have OA11. The economic burden of OA to individuals, the healthcare system, and society is significant12, with 18.9 (95% confidence interval (CI) 9.6–37.7) million years lived with disability (YLDs) globally7.
Farming is an occupation that includes heavy physical workloads13, and this can predispose a person to developing knee OA14-16. In the Canadian farming population, the prevalence of OA was 13% in Saskatchewan17, and 15% in the rural population of the neighboring province, Alberta18. Male farmers, who typically have a high physical workload, differed significantly from other work classes15,19,20. When compared to urban residents, Swedish farmers had a greater risk of developing OA (2.1; 95%CI 1.4-3.2)21, although this was based on a cohort of males aged 40–50 years, with a relatively short follow-up period of 13 years. An increased likelihood for both total hip (OR: 3.6; 95%CI 2.1 to 6.2) and knee replacement (OR: 5.1; 95%CI 2.1 to 12.4) was seen in male farmers in Iceland22. While OA has been examined in other occupations such as construction, carpeting, painting, fishery, and mining15,23, farming has often not been examined in spite of the high occupational demands associated with it.
Given that the susceptibility of developing OA is significantly affected by extrinsic risk factors including injury, and repetitive and excessive joint loading24, the risk of developing OA may increase in certain occupations14. The objective of this study was to estimate the annual incidence and mortality rates of OA among three random samples – Alberta farm, non-farm rural and urban residents – using provincial administrative health records over a period of 21 years. We also examined the hazards of developing OA among the farm and non-farm rural residents as compared to urban cohorts.
Methods
Study design and population
This was a longitudinal retrospective study that used provincial administrative data to identify OA cases over 21 years. Alberta, a Canadian province, had a farm population of 165 560 individuals in 200125. The main types of farming in Alberta include oilseed and grain farming (34.3%), beef and feedlots (20.9%), and dairy and milk farming (5%)26.
The study cohort consisted of 430 293 individuals, comprising three groups randomly selected: farm, non-farm rural, and urban residents. Alberta Health created a farm cohort based on the population registry in the fiscal year 1997–98. Through probabilistic matching with Alberta Agriculture and Rural Development and the Farm Fuel Tax subsidy, 143 431 farm family members of all ages with personal health numbers (PHNs) were identified. The Farm Fuel Tax file contained the names and addresses of farms and farmers that were eligible for farm fuel tax rebates from the Alberta government. Virtually all farms in Alberta that are involved in agricultural production qualify for this rebate. The non-farm rural cohort was generated with a random sample of 143 431 rural residents who were not in the farm group and their residence was located in a wide-area rural region. The urban group was a random sample of 143 431 urban residents who did not have postal codes that were for small towns or rural locations. As a closed population, no additional residents were added to the initial provincial cohort.
The inclusion criteria for this study cohort consisted of those who were 20 years or older during the fiscal years 2000–01 through 2020–21. Individuals aged less than 20 years who turned 20 years during any time during the observational period were included in the fiscal year they turned 20 years. Exclusion criteria were death, migration, or reaching 110 years of age. To optimize the identification of incident cases, a run-in period of 3 years (fiscal years 1997–98 through 1999–2000) was used to remove prevalent OA cases. After applying the general inclusion and exclusion criteria, 379 784 individuals were followed from 1 April 2000 to 31 March 2021 (Appendix I FigA1).
Data sources
Data were obtained from five provincial health administrative databases for fiscal years 1997–98 through 2020–21. Because Alberta has a universal healthcare system, all individuals have access to physician consultations, hospital treatment, and medical care27.
Alberta Health Care Insurance Plan (AHCIP) file: This contained all insured Alberta residents’ demographic information. The unique nine-digit PHN is used to link individual healthcare encounters, which includes all eligible medical benefits recipients during the fiscal year. Members of the Royal Canadian Mounted Police, members of the armed forces, prisoners in federal prisons, and Albertans who have not enrolled in the AHCIP are not included in this file.
Discharge Abstract Database (DAD) file: This consisted of hospital admissions, including the demographic information of patients receiving treatment, date of admission and discharge, diagnosis codes, intervention codes, and hospitalized time. All 25 diagnostic fields within the DAD were inspected for OA International Classification of Diseases, 9th and 10th revision (ICD-9 and ICD-10) codes.
Physician claims file: This included physician fee-for-service billing records, which comprised only a single diagnosis for each claim, represented by a three-digit truncated ICD-9 code, and included a PHN for each reimbursement.
National Ambulatory Care Reporting System (NACRS) file: This included outpatient medical and/or surgical services information provided by clinics, day surgery, and emergency room settings that receive public funds. Ambulatory care records, limited to 10 diagnostic codes, were collected for all patients with ICD-9 and ICD-10 codes related to OA across these fields.
Alberta Vital Statistics: This included date of death and primary cause of death (ICD-9 or ICD-10) from information on death certificates.
Case ascertainment
The case definition was used to identify OA cases in each of the three groups using the validated algorithm for OA28-30. This included at least one of the following criteria: one OA-related hospitalization with the OA diagnosis code (ICD-9: 715; ICD-10: M15 to M19 as first three digits), two OA-related physician visits within 2 years, or two OA-related ambulatory care visits within 2 years.
Statistical analysis
Descriptive statistics (mean, standard deviation (SD), or frequency count and percentage) were computed for relevant variables. Age was categorized into six groups (20–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years) based on the age of participants at the time of eligibility to the study. Socioeconomic status was also calculated based on the Income Support Flag in the AHCIP population registry file. If the flag was true, the socioeconomic status was considered to be low. The Romano comorbidity index was employed as a measure of comorbidity for chronic conditions diagnosed during the observational period31,32, 2 years prior and 5 years after the diagnostic date of OA.
Follow-up of each person was from the date of entry to the study period (the index date) and ended at the time when the person was first diagnosed with OA, reached the age of 110 years, died, migrated out of the province, or reached the end of study follow-up (March 2021), whichever occurred first. To eliminate prevalent cases, any previous history of OA diagnosis before 1 April 2000 was excluded. Incidence rate of OA was calculated for each of the three groups, and the risk of OA for both the farm and non-farm rural groups, relative to the urban group, was estimated using the crude incidence rate ratios (IRR) with 95% confidence intervals.
Trends of the OA incidence were calculated for farm, non-farm rural, and urban residents using Joinpoint regression analysis with Joinpoint software (v5.0.2)33. Joinpoint regression analysis was used to assess the magnitude and direction of temporal trends of the incidence for OA. Significant trend shifts known as joinpoints were identified while establishing linear trends. An annual percentage change (APC) is generated along with the average annual percentage change (AAPC) as a weighted mean of these APCs, thereby presenting a consolidated measure of the overall trend throughout the entire time.
The Kaplan–Meier method was used to calculate the survival probability considering censored data. To provide an estimated lifetime risk of OA, adjustment was made for mortality as a competing risk. While excessive mortality with OA is uncertain34, we calculated the impact of OA on the all-cause and non-injury mortality rate for each fiscal year based on the death registry records. Injury-related mortality cases were excluded to examine the impact of OA distinct from fatalities caused by traumatic events. The Cox proportional hazards model was used to estimate the hazard ratio (HR) for OA, adjusting for age, sex, and socioeconomic status based on residency status over a daily timeframe. The proportionality assumption for each comorbidity was tested by examining log–log Kaplan–Meier curves. Two-sided p-values were used for all analyses, with p<0.05 considered to be significant. All statistical analyses were conducted using SAS v9.4 (SAS Institute Inc; http://www.sas.com).
Ethics approval
The Health Research Ethics Board of the University of Alberta granted ethics approval for this study (Pro00091591).
Results
Of the 379 784 individuals who were followed from 1 April 2000, to 31 March 2021, 97 370 in the farm group, 91 543 in the non-farm rural group and 99,378 in the urban group were aged 20 years or older at the beginning of the study. During the observation period, an additional 35 153 individuals in the farm cohort, 28 617 in the non-farm rural cohort, and 27 723 in the urban cohort reached the age of 20 years and were included in the analysis. Within the study cohort, 51.6% (n=148 826) were male, and the farm group had the highest proportion of males (54.6%; n=53 194; p<0.001; Table 1). The mean age of the cohort was 45.1 years (SD 16.3 years), and the mean age of the farm group was greater than that of the other two groups (mean age 46.7 years; SD 15.9 years; p<0.001). The mean age at time of OA diagnosis was 64.7 years (SD 14.3 years) with no significant group differences (p<0.001). The farm group with OA had a mean Romano comorbidity index of 0.7 (SD 1.5) 2 years before the OA diagnosis, which increased to 1.8 (SD 2.6) in the 5 years following the diagnosis of OA. Non-farm rural and urban residents reported comorbidity indices of 0.9 (SD 1.6) and 0.8 (SD 1.6), respectively, 2 years before the OA diagnosis. Over the subsequent 5 years post-diagnosis, the comorbidities increased in both non-farm rural (2.1; SD 2.7) and urban (1.9; SD 2.7) residents.
During the 4 614 207 person-years (PYs) of follow-up, 67 387 people were diagnosed with OA in the study cohort. The overall crude incidence rate was 14.6 (95%CI 14.5–14.7) per 1000 PYs in the entire study cohort. Farm residents had the highest incidence rate (15.8 (95%CI 15.6–16.0) per 1000 PYs), followed by the rural cohort (14.7 (95%CI 14.5–14.9) per 1000 PYs) and the urban cohort (13.3 (95%CI 13.1–13.4) per 1000 PYs; Table 2). Physician claim records identified the most cases, with a case identification of 93.6%, of which 58.2% had health records related to OA exclusively in physician claims (Appendix I FigA2).
The overall incidence rate was 29% (95%CI 27–31%) higher in females (16.5; 95%CI 16.4–16.7 per 1000 PYs) than males (12.9 (95%CI 12.7–13.0) per 1000 PYs). The incidence rates in the farm group among males (14.3 (95%CI 14.0–14.5) per 1000 PYs) and females (17.7 (95%CI 17.4–18.0) per 1000 PYs) were higher than the incidence rates in the non-farm rural and urban groups (p<0.001). The incidence rate of OA increased with age regardless of group (p<0.001; Table 2). The farm group had a 19% higher risk of OA than the urban group (IRR 1.19; 95%CI 1.17–1.21), while the non-farm rural group had a 11% higher risk of OA compared to the urban cohort (IRR 1.11; 95%CI 1.09–1.13). In most cases, the farm group had a higher risk of OA than the urban group, regardless of sex (p<0.05; Table 3).
The crude incidence rate of OA over the 21-year period ranged from 19.1 (95%CI 18.6–19.6) per 1000 PYs in 2001 to 10.0 (95%CI 9.6–10.5) per 1000 PYs in 2021 (Appendix I Table A1). The joinpoint analysis revealed that OA incidence rate declined significantly in all three groups as determined by AACP (farm: –2.6, 95%CI –3.2– –2.1; non-farm rural: –3.1, 95%CI –4.0– –2.5; urban: –2.1, 95%CI –2.9– –1.6). The joinpoint model identified two change points in the trend of OA diagnosis in 2011 and 2019 for farm cohort and in 2010 and 2019 for non-farm rural and urban cohorts. From 2000 to approximately 2010, the overall (Fig1) and sex-specific (Fig2) incidence rates declined, whereas notable increases were then seen up to 2019 (p<0.05). Females consistently had higher incidence rates compared to males (p<0.001).
Among OA cases, non-farm rural residents had the highest all-cause (18.6 (95%CI 18.2–19.0) per 1000 PYs) and non-injury (18.0 (95%CI 17.6–18.4) per 1000 PYs) mortality rates, while farm residents had the lowest all-cause (13.5 (95%CI 13.2–13.8) per 1000 PYs) and non-injury (13.2 (95%CI 12.9–13.5) per 1000 PYs) mortality rates. Overall, males had higher non-injury mortality rates than females (p<0.05), regardless of OA status (Table 4). The Kaplan–Meier survival estimates showed that, during the 21-year study period, farm, non-farm rural and urban residents had survival rates of 72.5% (95%CI 72.0–73.1%), 67.0% (95%CI 66.4–67.7%), and 74.4% (95%CI 73.9–74.9%), respectively (Fig3).
After adjusting for mortality, the lifetime risks of developing OA were 27.7% for farm residents, 25.6% for the non-farm rural cohort, and 24% for the urban cohort. After adjusting for age and sex, the farm (6%) and rural (9%) residents had higher risks of developing OA as compared to the urban cohort (p<0.001) (Table 5). Females across all three cohorts had a 29% higher risk of developing OA (age and residency status adjusted HR: 1.29; 95%CI 1.27–1.31), and the risk of developing OA increased with advancing age.
Table 1: Baseline characteristics of the study population†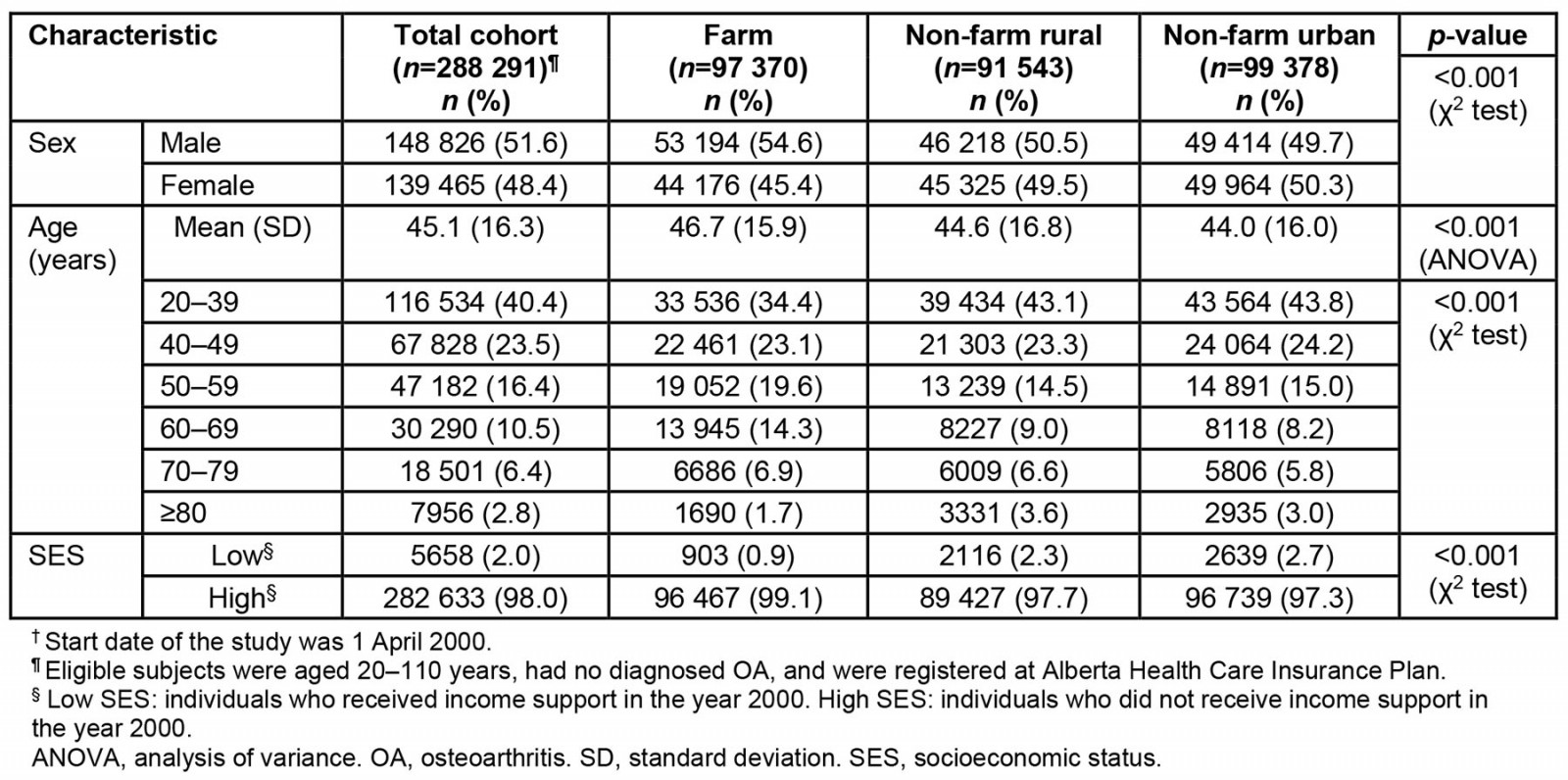
Table 2: Crude and age–sex-specific overall osteoarthritis incidence for the study population, 1 April 2000 – 31 March 2021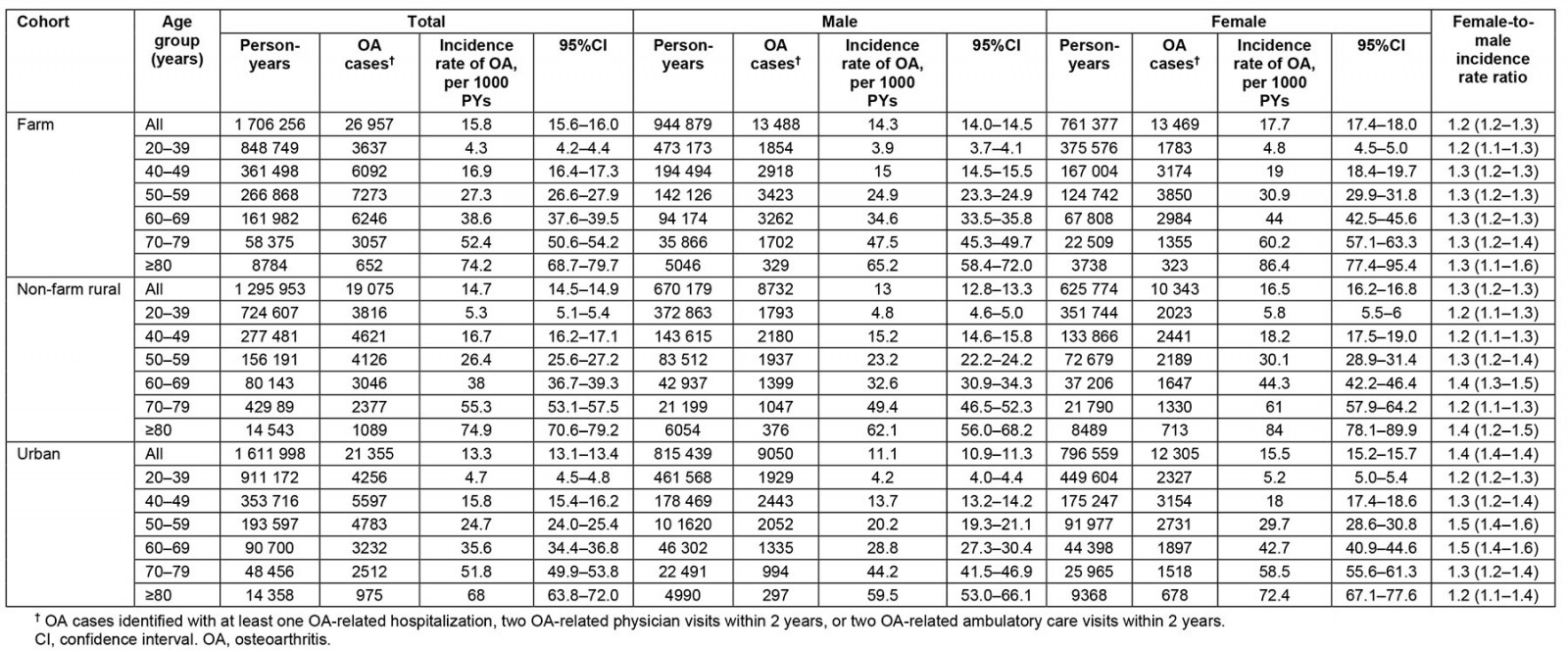
Table 3: Incidence rate ratio of osteoarthritis in farm and non-farm rural versus urban groups, 2001–2021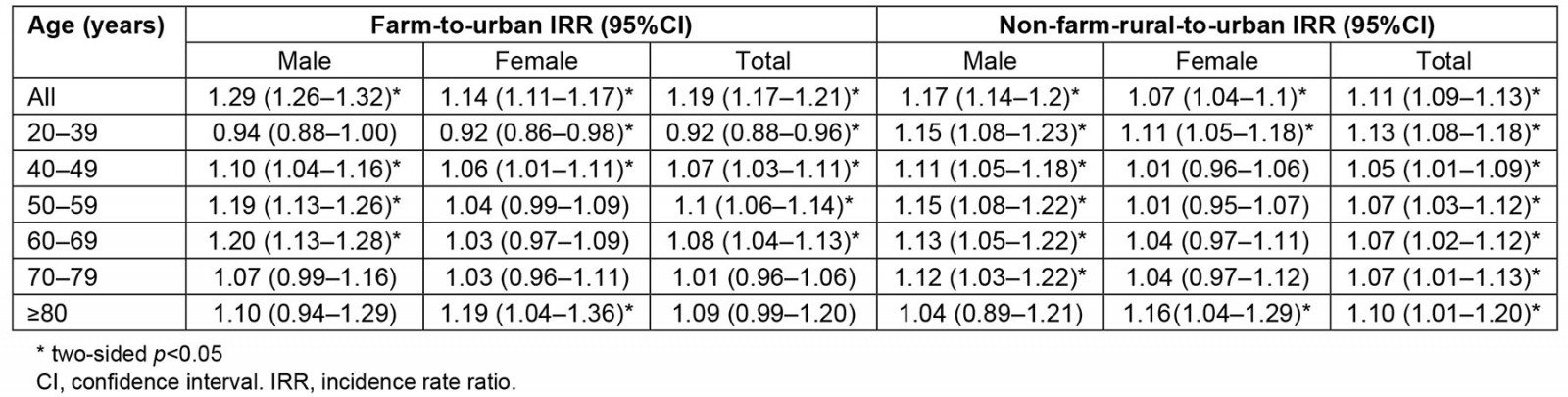
Table 4: Crude and sex-specific all-cause and non-injury mortality rate stratified by osteoarthritis in the farm, non-farm rural, and urban cohorts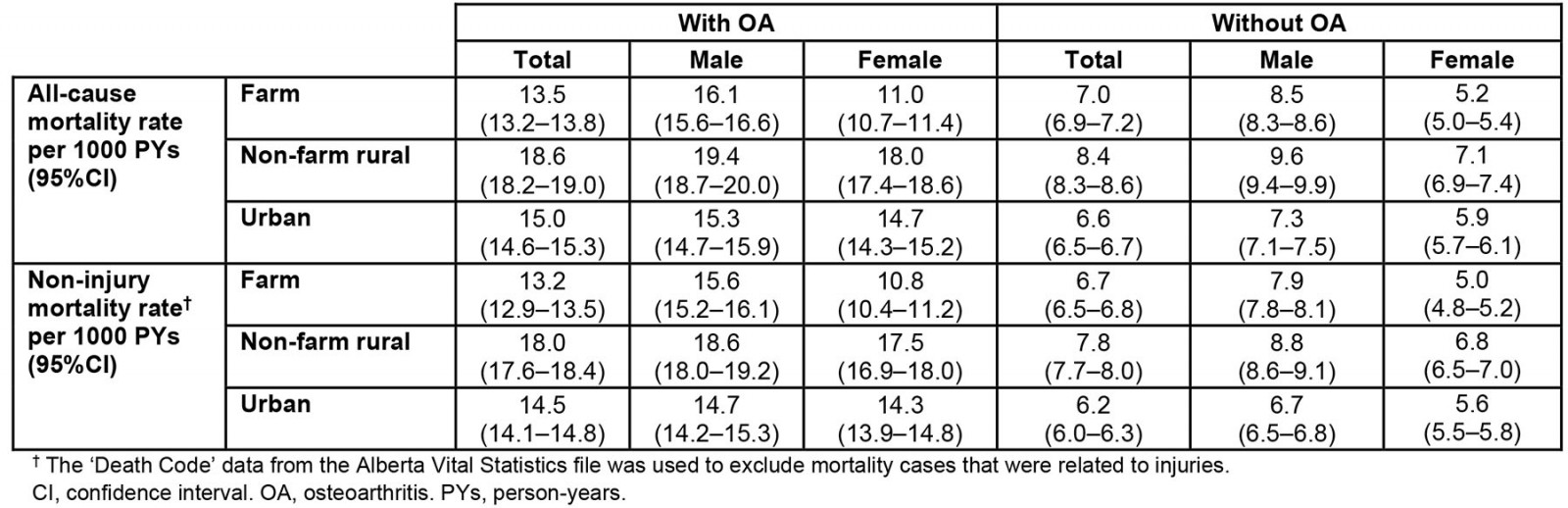
Table 5: Unadjusted and adjusted hazard ratio† for developing osteoarthritis based on residency status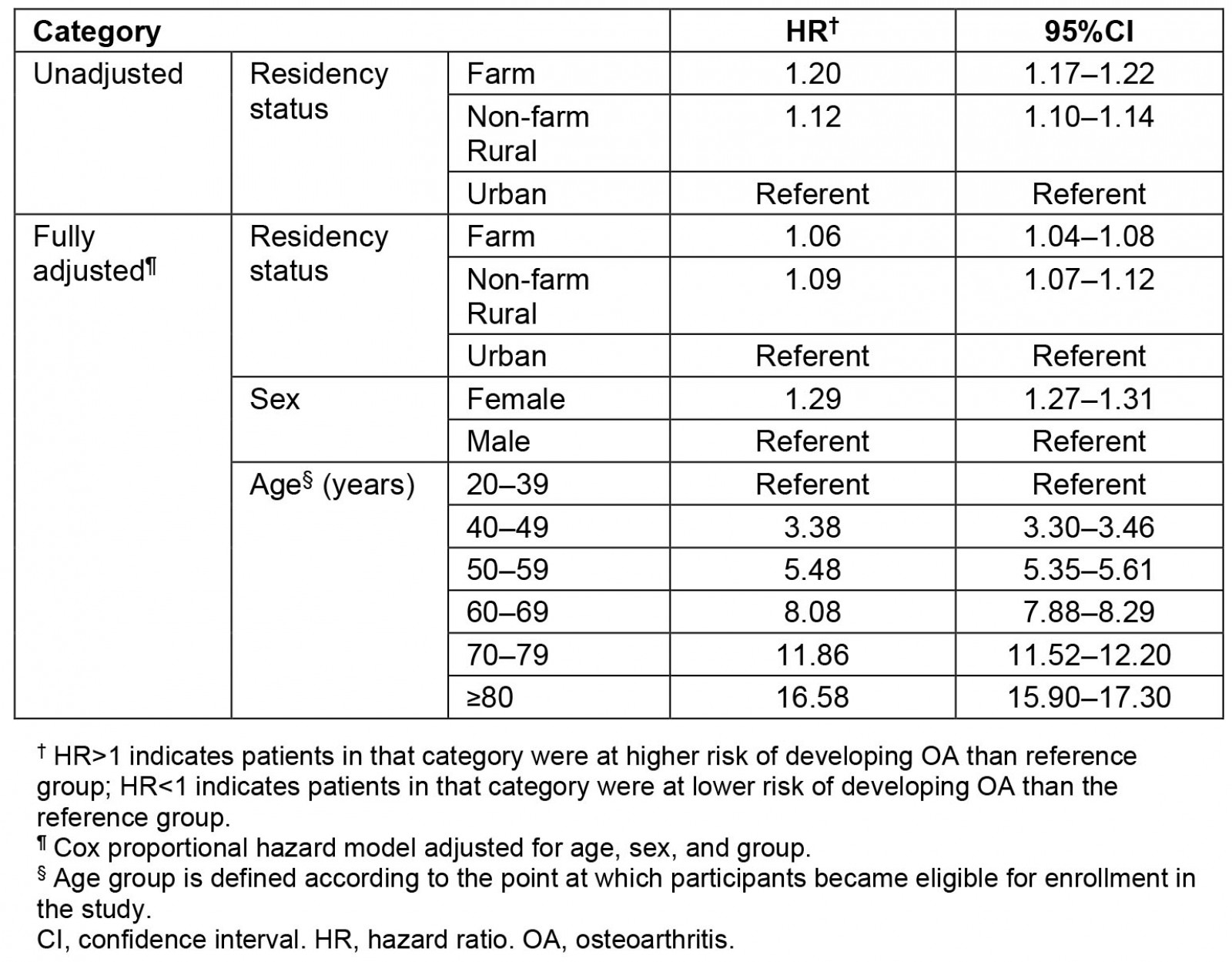
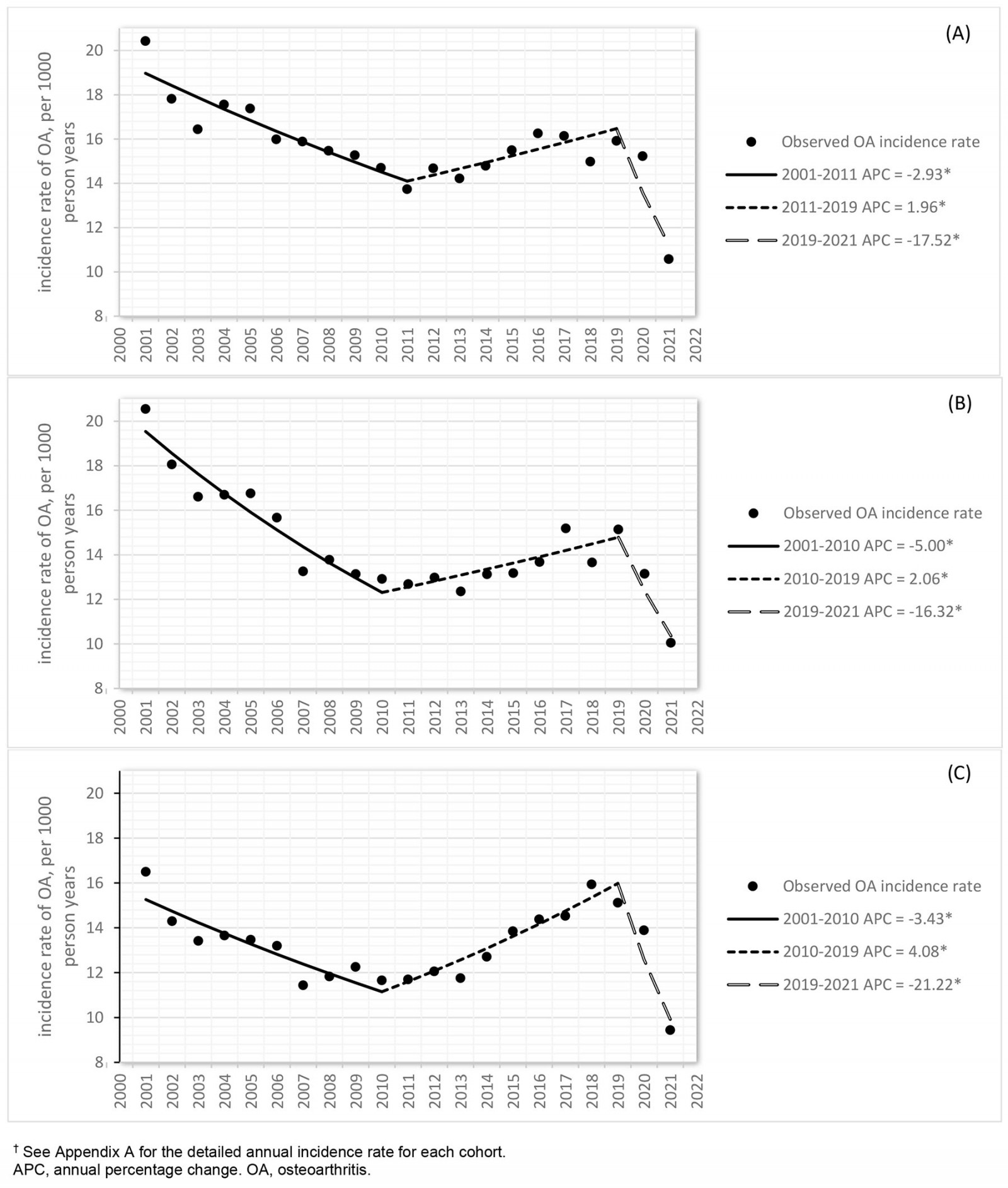 Figure 1: Joinpoint analysis of trends (p<0.05) in osteoarthritis incidence rate with annual percentage change for (A) farm, (B) non-farm rural, and (C) urban residents of Alberta, Canada, 1 April 2000 – 31 March 2021 (n=379 784).†
Figure 1: Joinpoint analysis of trends (p<0.05) in osteoarthritis incidence rate with annual percentage change for (A) farm, (B) non-farm rural, and (C) urban residents of Alberta, Canada, 1 April 2000 – 31 March 2021 (n=379 784).†
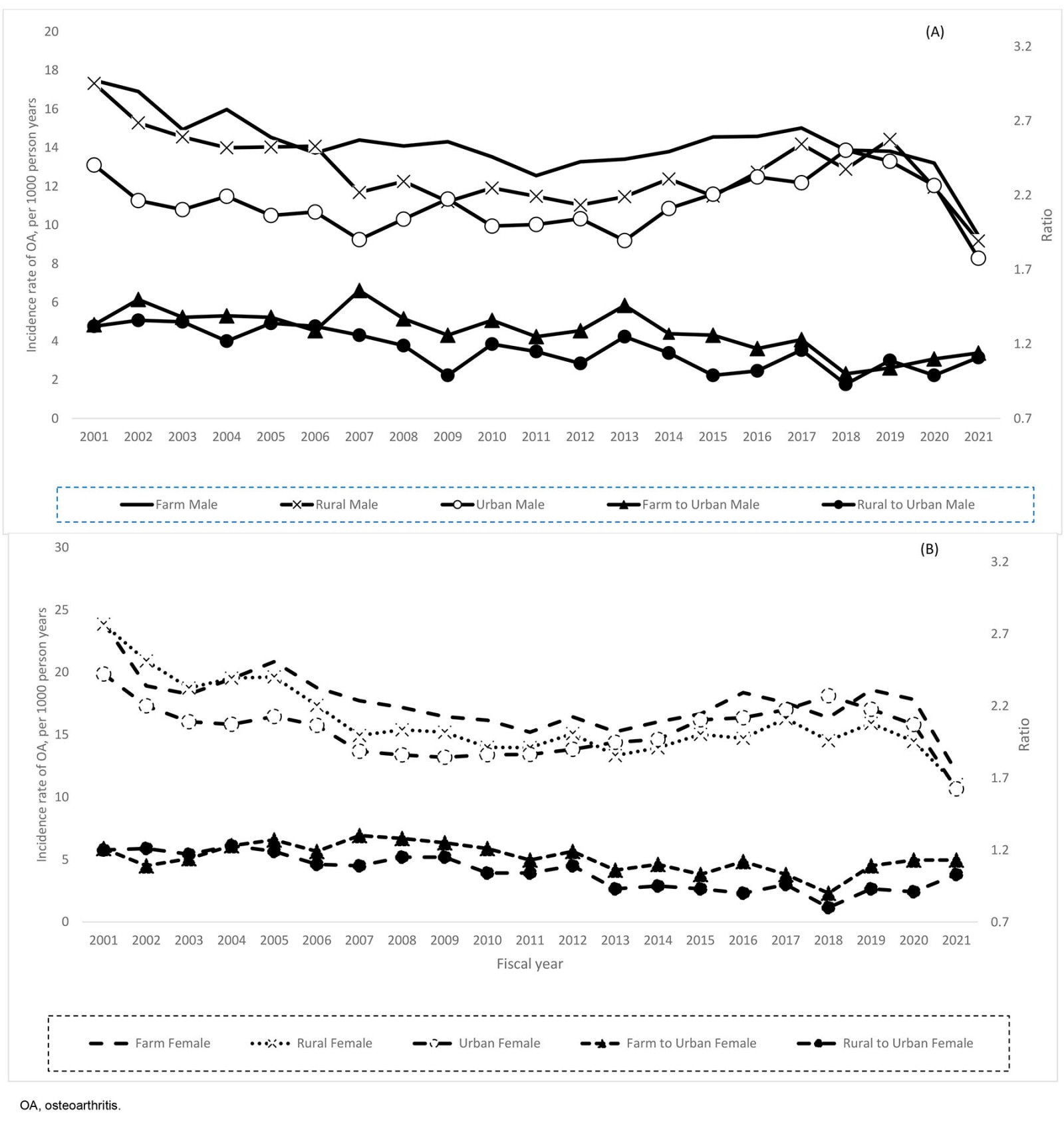 Figure 2: Sex-specific trends of annual incidence rate of osteoarthritis for (A) males and (B) females, 2001–2021.
Figure 2: Sex-specific trends of annual incidence rate of osteoarthritis for (A) males and (B) females, 2001–2021.
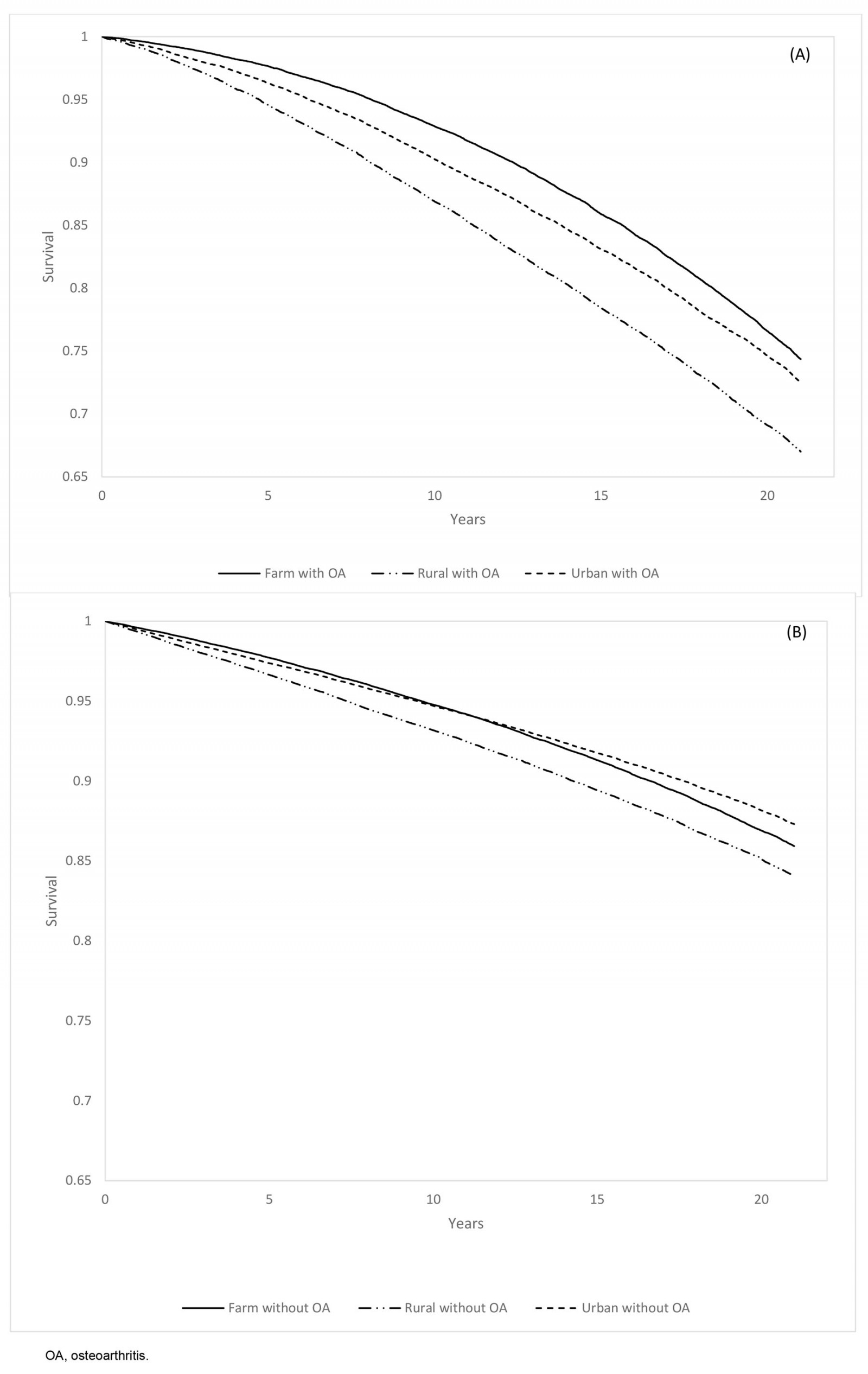 Figure 3: Kaplan–Meier curve for non-injury mortality rate in the farm, rural, and urban cohorts among individuals (A) with osteoarthritis and (B) without osteoarthritis, 1 April 2000 – 31 March 2021
Figure 3: Kaplan–Meier curve for non-injury mortality rate in the farm, rural, and urban cohorts among individuals (A) with osteoarthritis and (B) without osteoarthritis, 1 April 2000 – 31 March 2021
Discussion
Farmers and non-farm rural residents in Alberta had a higher incidence rate of OA than their counterparts residing in urban regions. Similar to other evidence, OA increased with age and was seen more often in females than males7,10,17. Over the 21 years, the rates increased regardless of being a farm, non-farm rural, or urban group. Lifetime non-injury mortality was greater for OA than non-OA regardless of group, while both farm and non-farm rural OA groups’ mortalities were greater with OA than the urban group. It cannot be discounted that group differences seen between the farm, rural, and urban groups may be a reflection of access to healthcare services including primary care35,36, and transportation barriers37,38. Although administrative data cannot discriminate the severity of OA, age at time of OA diagnosis for the farm cohort was older for than the other two groups, which may imply a greater number of comorbid conditions35,36.
Several studies have examined occupational activities23,39 and reported that those in physically demanding occupations such as farming are at increased risk for developing OA. A cross-sectional study conducted in Saskatchewan, which has similar farming practices to Alberta, reported 10.2% (95%CI 8.9–11.5) of farm residents had physician-diagnosed OA17. A study on Swedish farming that included crop cultivation, forestry, and dairy farming reported that the risk of developing OA was 2.1 (95%CI 1.4–3.2) times greater among male farmers aged 40–60 years than the urban males of the same age group21. A cross-sectional study conducted in rural Britain found that long-term farming had a higher prevalence of hip OA compared to individuals with sedentary jobs, showing an odds ratio of 9.3 (95%CI 1.9–44.5)40. Varying types of farming and farming practices in different countries may, in part, explain the varying rates reported with incidence and prevalence of OA in farm cohorts41.
A systematic review reported strong evidence for developing hip OA with lifting activities, while moderate evidence supported cumulative effects of physical loads and full-body vibration with hip OA risk39. Most of the articles included in the review were classified as multiple industries rather than specific occupations including farming. The authors raised key challenges in that measures to assess occupational activities and recall periods were limited39. Andersen et al similarly reported a higher risk of developing hip OA (HR: 1.63; 95%CI 1.52–1.74) among male and female Danish farmers compared to those in more sedentary occupations such as office work42, while in our study the farm population included all farm family members based on the probabilistic match of Alberta Agriculture and Rural Development with the Farm Fuel Tax subsidy.
Our results indicate that non-farm rural residents are at higher risk of developing OA compared to urban residents. A significant portion of non-farm rural residents are involved in various occupations that require considerable physical exertion, as evidenced by data from the 2019 Census. Construction, transportation and warehousing, agriculture, forestry, fishing, hunting, and manufacturing are the predominant occupational domains within rural communities43. These activities can be labor-intensive, particularly during the busiest seasons, and may require manual labor for long periods. This observation underscores the significant impact of different physically demanding occupations on OA risk within rural residents42,44,45.
Over time a similar pattern of incidence was seen in the three groups. The joinpoint analysis indicated a decrease in OA incidence rate from 2001 to 2011, followed by an increase until 2019 across all three cohorts. This fluctuation is consistent with findings of other studies10,46. The trend in incidence rates within an administrative database for OA relies on the number of run-in years utilized to remove prevalent cases47. While we excluded prevalent cases using administrative data with a 3-year run-in period, the initial years might have overestimated annual OA incidence rate by not identifying prevalent cases within that time frame in the administrative data. Using a 9-year run-in period, Rahman et al noted an increasing trend in crude incidence rates for both men and women in British Columbia48.
The annual incidence rate for all three groups dropped sharply after 2019, possibly due to the impact of the COVID-19 pandemic. The pandemic resulted in the diversion of many healthcare resources and staff to treat people with COVID-19, leading to a shortage of supplies and significantly reduced access to medical care49. This reduced access to health services, and the fear of contracting COVID-19 potentially contributed to the observed decrease in OA incidence rates50,51.
OA is the leading cause of disability7, particularly walking disability – a disability associated with reduced walking frequency and linked with premature mortality in older adults with OA52. The sedentary lifestyle and diminished physical activity significantly contribute to the development of various medical morbidities, including metabolic diseases, cardiovascular disease, and an increased risk of mortality53,54. Our findings showed a similar pattern in all-cause and non-injury mortality rates, with notably higher rates among individuals with OA compared to rates for those without the condition. This trend aligned with factors including a higher comorbidity index, more functional limitation mobility, and higher disability-adjusted life years (DALYs) among individuals with OA7,55-57. Of more notable interest, farm residents with OA had the lowest all-cause and non-injury mortality rates compared to those of other groups. Regardless of OA status, non-farm rural residents had the highest mortality rate. While the evidence is inconclusive regarding the relationship between OA and mortality58, others have recognized potential confounding effects in explaining mortality and OA. Sedentary activity is a risk factor for mortality, and the lower mortality rate reported in the farm group may be related to activity associated with farming. Further investigation is warranted with the risk factors, OA and mortality in occupational activities.
The use of administrative data for the development of chronic diseases is useful to derive population-based estimates; however, there are inherent limitations of administrative data that need to be recognized. Our case definition was based on healthcare visits, whether for primary care or hospitalizations28,29,59, not when signs or symptoms first appeared. OA is a chronic condition that develops over years, and several individual factors such as obesity, age, and genetics9,60,61, and availability of health services, need to be considered as to when a person will seek medical attention.
The type of joint affected by OA could not be identified with this data. Access to joint-specific data and more detailed information on various physical activities performed – such as lifting, kneeling, squatting, along with specific details on their intensity and frequency – would significantly enrich and broaden our understanding of how farming practices may relate to OA development. Another consideration is that the farm group comprised family members based on the probabilistic match of Alberta Agriculture and Rural Development with the Farm Fuel Tax subsidy; however, we restricted the cohort to those aged 20 years or older. The amount of farming activities is not known, yet others have acknowledged the complexity of work activities and OA and have called for more direct measures such as technology and video assessments to be used for work activities39.
Lastly, the healthy worker bias cannot be disregarded39. Farmers experiencing joint pain might opt to leave their occupation prematurely. This departure could result in an underestimated incidence rate of OA among farmers. Acquiring data detailing the work intensity both before and after leaving farming roles is essential to comprehensively understand the impact of work on OA occurrence.
Conclusion
Among Albertan residents, the risk of developing OA among Albertan rural residents, including both farm and non-farm populations, is higher compared to the urban population, who more often have sedentary occupations and can readily access healthcare services. As further research is warranted in occupational-related OA, farming is an occupation with several occupational exposures that have yet to be described or measured. Others have called for more stringent methods of measurement and recall of work activities (eg frequency, intensity, and duration) to inform prevention strategies and policies in occupational health39. Physically demanding working conditions such as farming are public health concerns in which practice strategies and access to healthcare services in rural communities need to be highlighted in the management of OA.
Acknowledgements
The authors thank Alberta Health for providing data for cohorts of this study. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta. Neither the government nor Alberta Health express any opinion in relation to this study.
Funding
No funding was received for this research.
Conflicts of interest
The authors declare no conflicts of interest.
References
appendix I:
Appendix I: Annual osteoarthritis (OA) incidence rate per 1000 person-years (PYs) from 2000-2001 to 2020-2021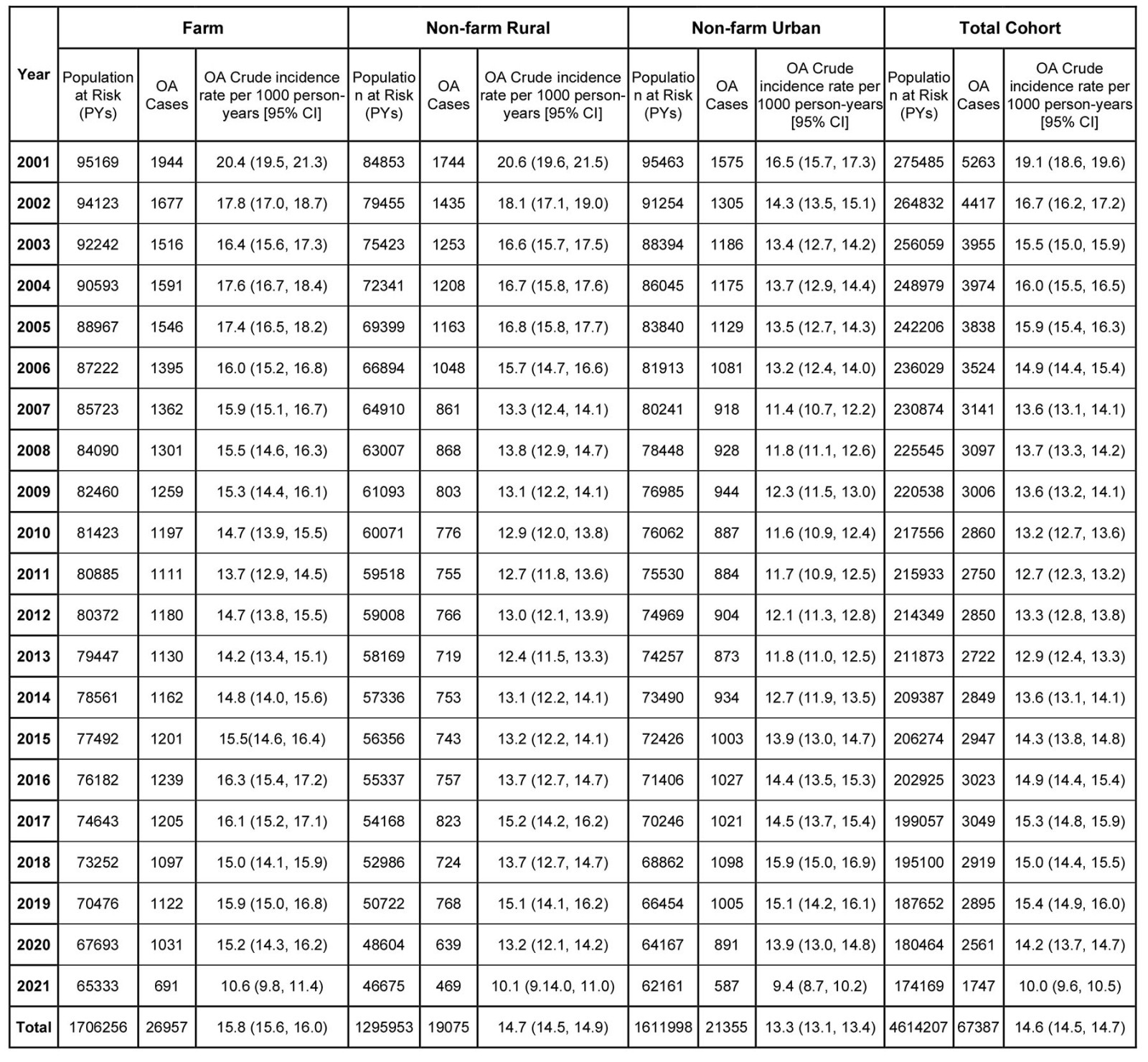
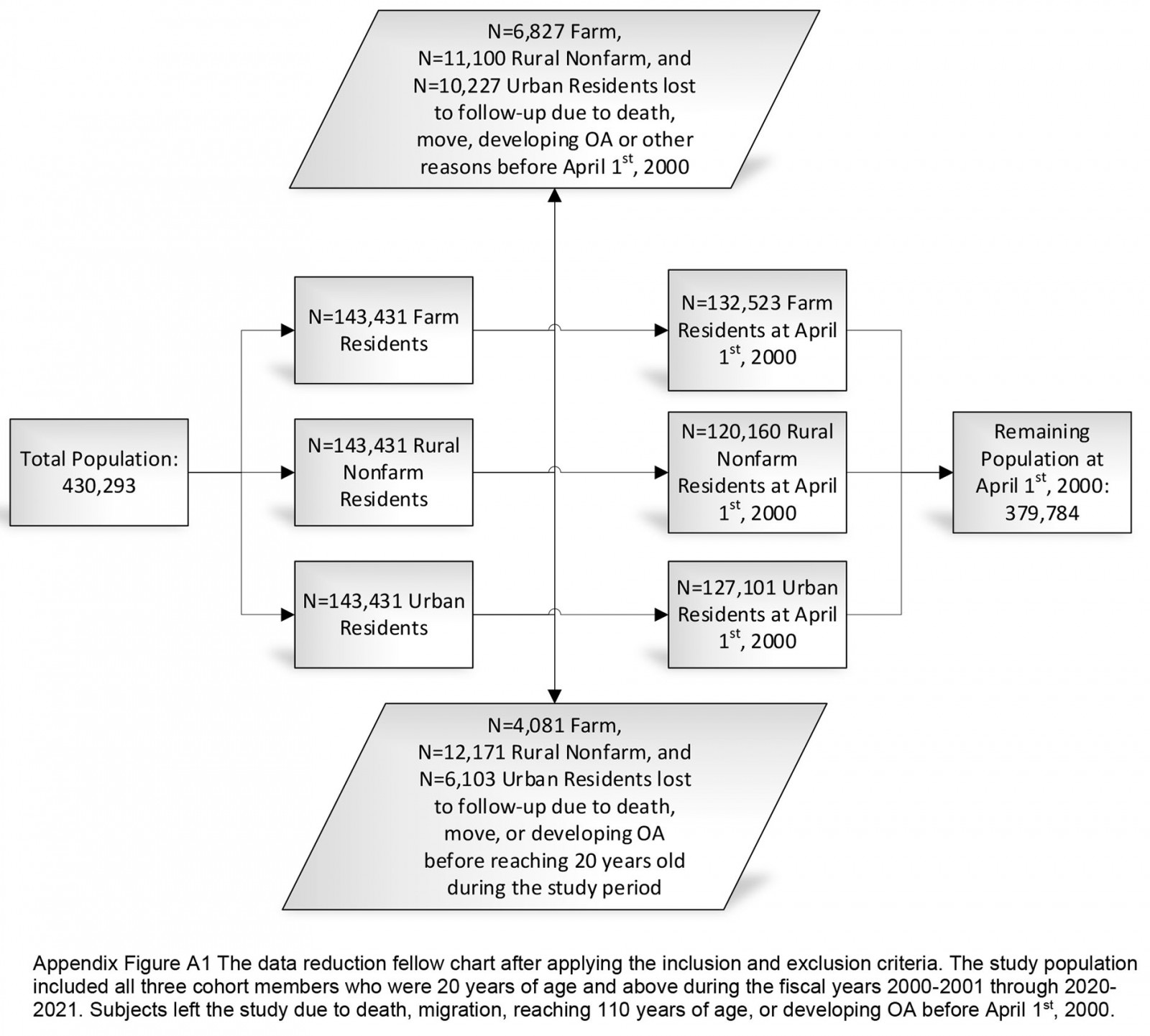 Appendix I FigA1
Appendix I FigA1
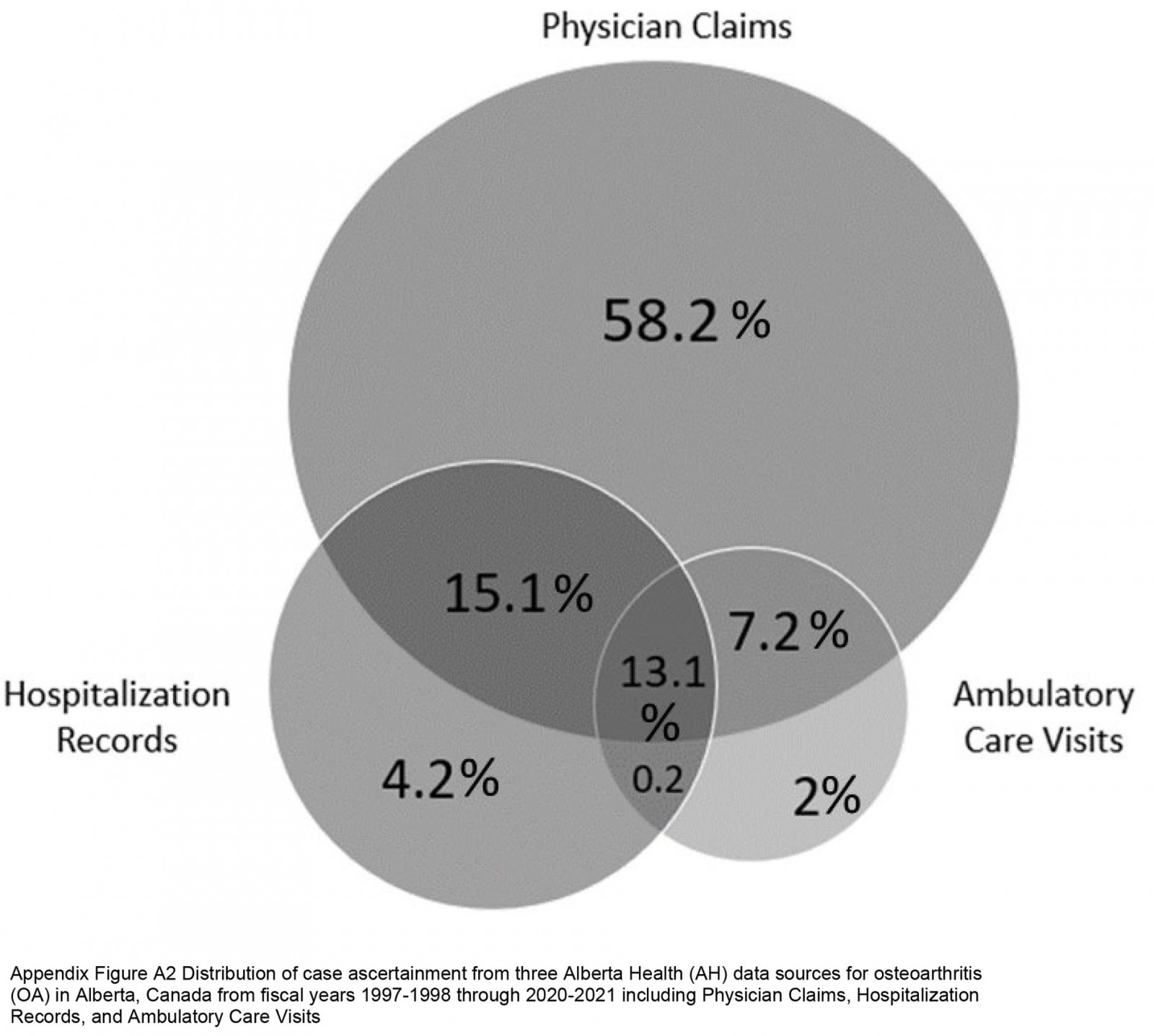 Appendix I FigA2
Appendix I FigA2
You might also be interested in:
2019 - Appearance of ureterorenal stones after gouty arthropathy: ethinic disparity
2011 - Idaho Rural Family Physician Workforce Study: the Community Apgar Questionnaire



