Introduction
Skin cancer is a major public health issue in Australia, with the highest incidence of skin cancer globally1. Melanoma in particular is renowned as ‘Australia’s national cancer’1. Non-melanoma skin cancer (NMSC) is the cancer most commonly diagnosed in general practice, both in Australia and globally. NMSC frequency is five times that of all other cancers combined, resulting in a substantial economic burden to the Australian economy of approximately $1.7 billion annually1,2.
Rural Australian populations experience a higher skin cancer burden when compared to their metropolitan counterparts3,4. While lifetime risk of diagnosis of melanoma skin cancer and NMSC is increasing, the mortality for both types of skin cancer remains low5,6. Rural populations have a higher melanoma mortality rate when compared to metropolitan areas7. Those living in the state of Victoria and outside major cities are 44% more likely to be diagnosed with melanoma, with the highest likelihood of diagnosis in the South West Victoria region8. Given NMSCs are not reportable conditions, it is difficult to ascertain whether the same trends exist. These differences in incidence and mortality have been attributed to a combination of an ageing rural population, the increased occupational risk from UV exposure (such as for farmers in rural regions), and inequities in service availability between metropolitan and rural areas7.
Australian GPs manage over half of Australia’s skin cancer cases, constituting approximately 3% of all GP patient presentations9,10. Patients experiencing socioeconomic disadvantage, and rural Australians, are more likely to have their skin cancers diagnosed and managed in primary care, reflecting the financial and geographical accessibility of GPs10. As of the 2021/2022 medical workforce analysis, there were 39 259 GPs nationally, of whom 9629 practised in Victoria and 416 were practising in rural locations11. GPs are responsible for investigating, diagnosing and managing the array of skin presentations in rural areas as approximately 92% of specialised dermatologists are based in metropolitan centres12. While skin cancer detection is included in the Royal Australian College of General Practitioners’ training curriculum, there is a paucity of formalised training, with clinicians relying on external courses or on-the-job training5,13. As a result, considerable variations have been identified in GP confidence in diagnosis and management of skin cancers, largely due to the requirements of GPs in rural settings to be multi-skilled, variable opportunities to participate in specialised training and those with a particular interest incorporating skin cancer as a speciality in their practice5. GPs able to accurately detect skin cancer early minimised both the morbidity and mortality burden, with delays in diagnosis of stage I melanoma increasing the mortality risk by 5%14-16.
Existing literature has covered the epidemiology of skin cancer in rural populations across various states, yet there is limited research specifically focused on Victoria17. This clinical review addresses this gap by investigating the detection of melanoma skin cancer and NMSC in a rural general practice in the South West region of Victoria. The clinic location is categorised as a medium rural town by the Modified Monash Model (MM 4), but also serves a broader agricultural area of small rural towns (MM 5), serving a population of about 16 50018.
Methods
This clinical review was a retrospective data audit of patient files for two different time periods: 14 October 2019 to 5 November 2020 and 2 February 2021 to 20 February 2022. Due to the impact of the COVID-19 pandemic, the time periods were unable to be exactly 1 year apart. A retrospective study design provides an efficient process in data analysis and is a practical methodology in terms of managing datasets19.
Participants and data collection
A file review was completed in the clinic’s electronic medical records (ZedMed) of all Medicare item numbers billed for skin cancer and presumed skin cancer excisions (items 31356 to 31376) for all doctors for the periods of 14 October 2019 to 5 November 2020 and 1 February 2021 to 17 February 2022. Clinical information was cross-checked with hard-copy treatment room records.
Data collection focused on complete excisions of skin cancers only, not on any lesions that were treated non-surgically after a diagnostic biopsy. Neither punch nor shave biopsies were included in the dataset, to streamline data collection.
Lesions that were re-excised where surgical margins were inadequate were only counted as a single procedure. Multiple excisions from different anatomical locations from the same patient were counted as separate procedures.
Statistical analysis
Data were reported as mean, range and standard deviation (SD) where appropriate. All p-values less than 0.05 were considered statistically significant. Statistical analysis was conducted using STATA v17 (StataCorp; https://www.stata.com) (Tables 1–3).
Number needed to treat (NNT) is a measurement of the impact of medicine or medical intervention by estimating the number of patients that need to be treated in order to have an effect on one person, and it is a common metric for evaluating skin cancer detection13.
When applied to skin cancer detection in this retrospective analysis, NNT is a ratio calculated to determine the total number of patients that need to be treated, to prevent one incidence of skin cancer, calculated individually for each cancer, thus representing the ratio of benign skin lesions excised for every confirmed malignancy13,20. It was calculated by dividing the total number of lesions excised by the number of malignant lesions excised and rounded to the nearest whole number.
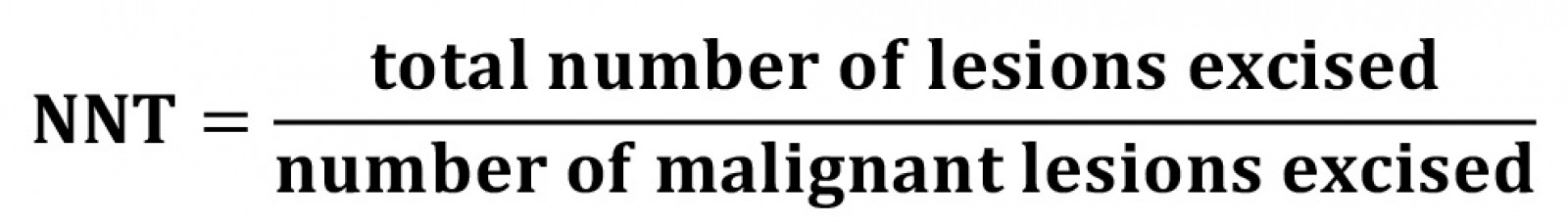
In the setting of this retrospective research, the number needed to treat (NNT) is a concept to provide a measurement of diagnostic accuracy in melanoma detection, where the ratio reflects the number of benign pigmented lesions removed for each melanoma13. It is suggested that the lowest NNT results are associated with dermatologists, followed by GP skin cancer specialists20. A lower NNT has been linked with improved patient care by aiding in earlier detection and reducing unnecessary biopsies13,20.
Diagnostic accuracy was calculated to assess how experience affects the pre-procedural diagnostic ability of each clinician. In this context it is a more specific measure than NNT as it calculates if a clinician’s exact pre-procedural diagnosis was in keeping with the histology report. Where NNT is a more general measure, diagnostic accuracy provides context on how experience and diagnostic skills are linked.
A diagnosis was found to be correct if the preoperative notes suggested a lesion was a basal cell carcinoma (BCC) and the histology confirmed a BCC. If the pre-procedural diagnosis was a squamous cell carcinoma (SCC) and the lesion was histologically a BCC or a melanoma, this was still graded as inaccurate.
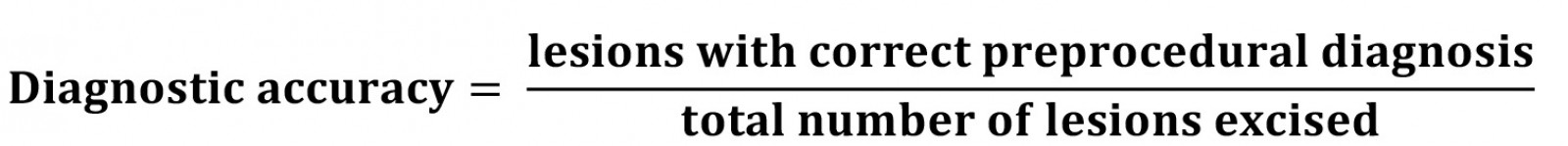
A companion article explores administered anaesthetic (volume/dose), complications (dehiscence/infection), patient and clinician demographics over the same time periods21.
Ethics approval
Ethics approval was granted by Deakin University Human Research Ethics Committee (DUHREC) (approval number 2022-177).
Results
Across the study periods, a total of 789 lesions were excised from 575 patients, of which 449 (56.9%) lesions were histologically confirmed to be malignant (Table 1). Patient ages ranged between 17 and 99 years, with mean ages for men and women of 68.3 and 65.5 years, respectively. In male patients 62.2% of lesions excised were histologically confirmed to be malignant, while in females the proportion of malignant lesions was much lower, at 49.7%. There was a corresponding statistically significant difference in malignancy rates between genders (p≤0.001).
Preoperatively, 681 lesions were suspected to be malignant. Table 2 compares the preoperative clinical diagnosis with the histological diagnosis. The preoperative diagnostic category ‘aesthetic’ refers to lesions the clinician believed to be benign but the patient wanted removed for cosmetic purposes. Overall, GPs were mostly correct in identifying malignancies, especially for NMSCs, whereas melanomas were more difficult to correctly identify. Regarding NMSCs that were correctly identified, BCCs were diagnosed 63.8% of the time and SCCs 59.1%. Melanomas were only identified correctly 15.6% of the time; however, considering the difficulty in diagnosis, this is expected19,22.
There were similar numbers of excisions across the head and neck, trunk and limbs (Fig1). NMSCs were more commonly removed from sun-exposed areas of the body such as the head and neck, and limbs. There were differences between the locations of SCCs and BCCs. BCCs had a greater occurrence on the head and neck, while SCCs were more common on the limbs. In contrast, melanoma was predominantly found on the trunk.
A total of 15 GPs removed lesions, with experience ranging from registrar level to specialist GP skin practitioners. Table 3 presents the number of removals and diagnostic accuracy by years of experience in general practice. Using the aforementioned NNT calculation approach, the NNTs for melanoma and NMSC were 5.4 and 1.4, respectively.
Table 1: Skin cancer type by patient gender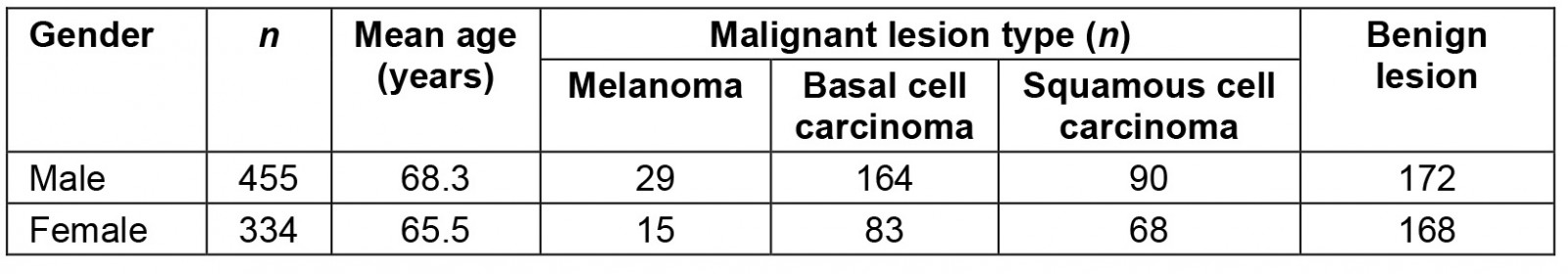
Table 2: Histological skin lesion diagnosis compared with preoperative diagnostic category in a rural clinic in South West Victoria, Australia, 14 October 2019 – 5 November 2020 and 1 February 2021 – 17 February 2022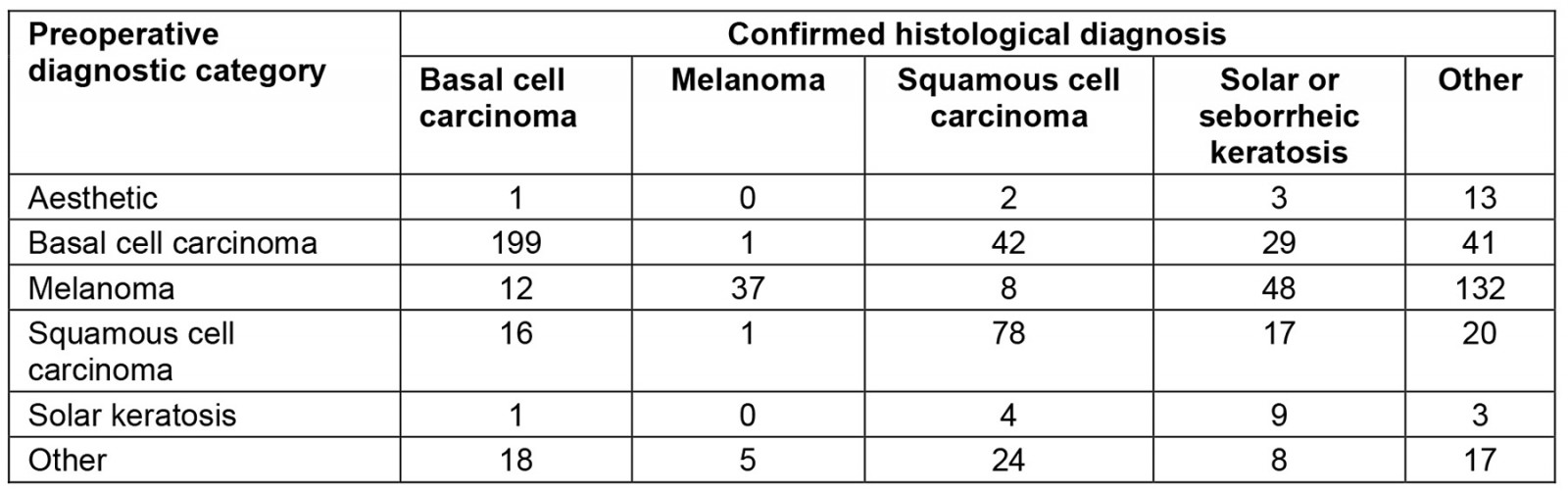
Table 3: GP diagnostic accuracy compared to years of general practice experience in a rural clinic in South West Victoria, Australia, 14 October 2019 – 5 November 2020 and 1 February 2021 – 17 February 2022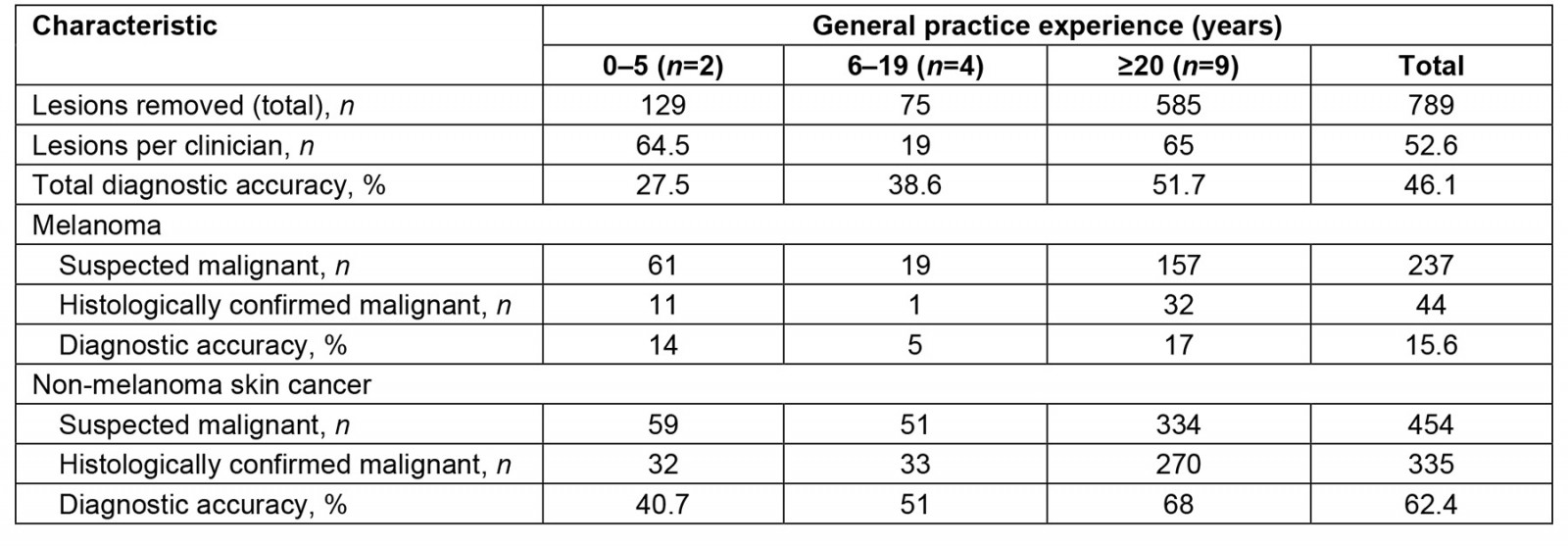
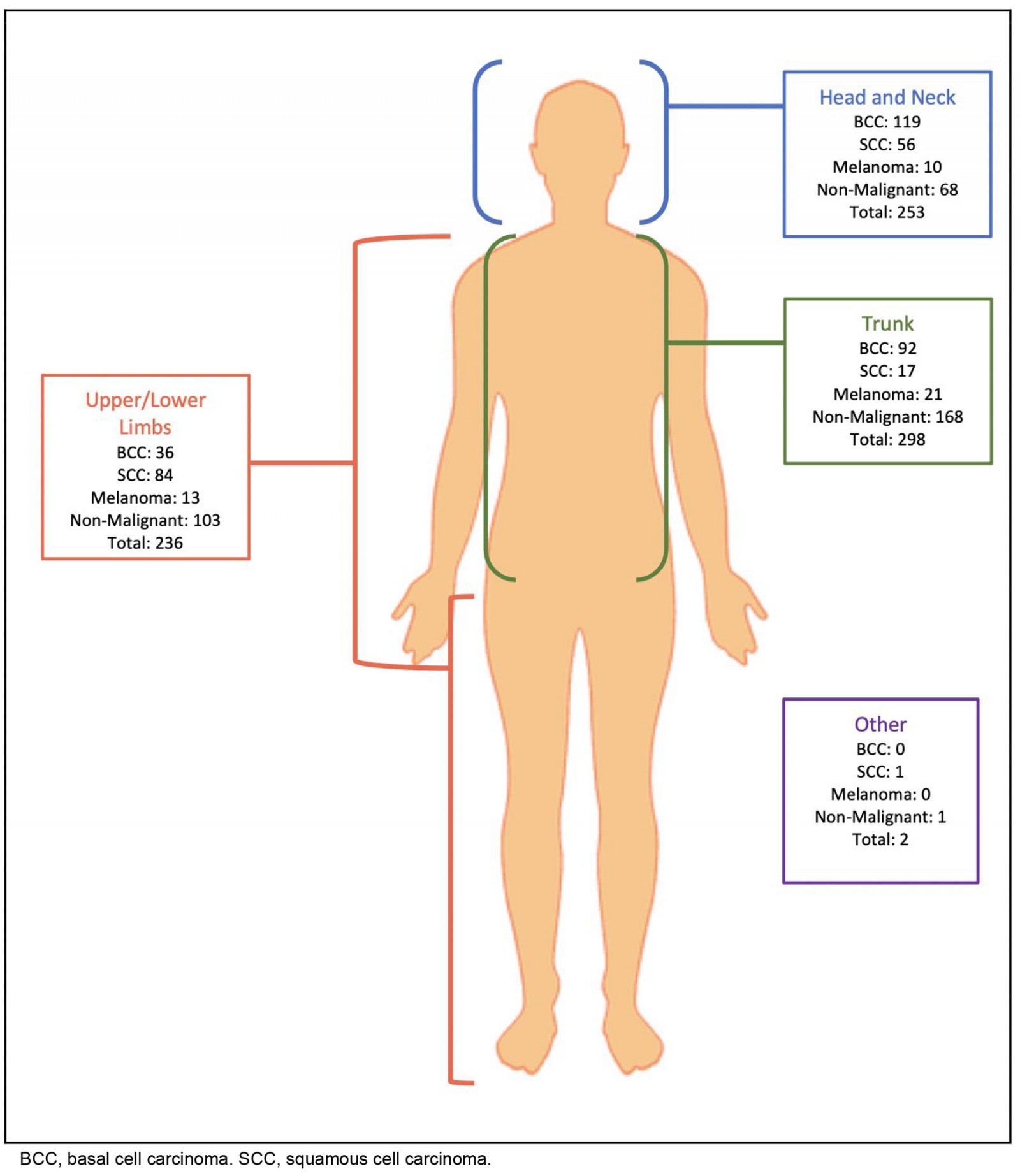 Figure 1: Location and histology of excised lesions.
Figure 1: Location and histology of excised lesions.
Discussion
This study examined the NNT and incidence of skin cancer in a rural general practice in South West Victoria. The location is in an MM 4 location that also services a wider agricultural geographic area of MM 5 due to the scarcity of primary healthcare services in the area.
Epidemiology
A total of 789 lesions were removed from 575 patients across two distinct time periods. The data were collected as part of a broader investigation into skin cancer excisions.
A total of 56.9% of excisions were histologically confirmed to be malignant. The anatomical distribution of skin malignancies aligned with trends reported in existing research2,23,24.
Males were significantly more likely to be diagnosed with skin cancer. Notably, there was an over-representation of males diagnosed with melanoma compared to the statewide average25. This difference occurred despite the practice catchment exhibiting a gender ratio comparable to the statewide figure26. While comparative data for NMSC are unavailable due to it not being a reportable condition, it is known rural males are significantly more likely to be diagnosed with cancer than metropolitan males27.
The higher rates of skin cancer among rural males may be attributed to the occupational profile of the rural workforce, with a higher percentage of male farmers and outdoor workers7. Additionally, rural males are more likely to recognise personal skin cancer risk factors, self-examine their skin and seek skin checks than their metropolitan counterparts20,28,29.
Melanoma and non-melanoma skin cancer
This study determined the NNT for melanoma is 5, a significantly lower figure than for other research findings13,29,30. Australian literature, focusing on skin cancer detection in primary care, estimates the NNT range for melanoma to be 9.4–4013,28,29, while an international meta-analysis estimated the NNT for melanoma was 20,22,2320. The broad range in melanoma NNT across the literature reflects the diversity within the GP workforce. GPs with a special interest in skin cancer are often those working in areas with a higher prevalence of skin cancer, achieving a lower NNT13,20,28. The prevalence of skin cancer in regional Australia is another factor contributing to improved NNT due to increased caseloads and exposure frequency13,30-33.
As expected, the NNT for NMSC was lower than for melanoma. This is due to the challenge in distinguishing subtle skin changes associated with melanoma. Furthermore, melanomas are less common and, due to their high mortality risk, clinicians often adopt a more conservative approach19,33. The calculated NNT for NMSC was 1, which aligned with other Australian studies, with an NNT range for NMSC of 1.5–1.729,34.
While there is no ideal NNT, a lower value is often preferred as it generally reflects a higher diagnostic accuracy, fewer unnecessary excisions and a reduction in associated morbidity and economic burden29,30. However, a low NNT may signify too narrow an excision criterion being used, resulting in under-excision of lesions30.
The low NNT for both melanoma and NMSC in this study may be attributable to the increased experience of rural GPs, due to the locality and specialist maldistribution of the Australian workforce. National data reports the number of GPs per 1000 people is highest among MM 4 locations, at 1.3 per 1000, but this decreases to 0.8 per 1000 in MM 5 locations35. Clinics such as the one in this research are within an MM 4 region. MM 4 areas often have a larger ratio of catchment area to population and consequently have more scope and responsibility than their metropolitan counterparts9,12.
There are significantly fewer non-GP specialists in MM 4 and MM 5 locations, at 0.4–0.1 per 1000, 4.5 times lower than in MM 3 locations35. While these statistics are nationwide and do not account for the heterogeneity within rural Australia, they do provide insight into the increased and broad experience rural GPs develop compared to their urban counterparts.
GP diagnostic accuracy
This study used diagnostic accuracy to evaluate its relationship with GP experience. Diagnostic accuracy, rather than NNT, was used as it allowed comparison between pre-procedural and histological diagnosis, providing a more specific reflection of diagnostic skill.
Our results indicate a positive association between increasing GP experience and diagnostic accuracy, and this result aligns with previous research13,20. GPs with more than 20 years of experience demonstrated the highest diagnostic accuracy for melanoma skin cancer and NMSC, at 17% and 68%, respectively. Interestingly, GPs with 6–19 years of experience exhibited a lower diagnostic accuracy for melanoma compared to their less-experienced counterparts. In contrast, those with 0–5 years of experience showed an interest in skin cancer management but lacked the general practice medicine expertise of more senior colleagues. In existing literature, there is substantial variability in the reported diagnostic accuracy for GPs13,20,32. Determining the true diagnostic skill within the workforce is difficult due to the broad range of clinician interest, training, clinical exposure and patient demographic characteristics.
GPs with 6–19 years of experience conducted the fewest procedures within this study. This is attributed to their clinical area of interest, and their demographic characteristics. While it is known that having a special interest in skin cancer increases a GP’s diagnostic accuracy, the impact of clinical interest on the availability of timely skin cancer care is not well documented36,37. This highlights a potential area of further research as there are increasing numbers of skin cancer presentations, often outweighing practitioner availability, especially in rural settings where health service capacity is stretched38-40.
Limitations
Several limitations within this study have been identified. It is unclear whether these results are transferrable to other rural settings in Australia, as there is considerable variation in healthcare availability, climate and demographics across the country, particularly in rural MM 4 and MM 5 settings. Moreover, this study was a subset of a larger intervention study that spanned two discrete time periods. While the intervention was not relevant to the data used, it is not known whether it resulted in a subconscious change in behaviour in GPs. As the larger study did not include shave or punch biopsies or delineate between in-situ and invasive lesions, these data were not available. Due to the retrospective nature of this research, some data may have been incomplete and absent due to inconsistent documentation available. Also, this study design did not account for patients who may have been referred to local surgeons or out-of-town dermatology services, which may potentially impact the estimates of NNT and diagnostic accuracy.
Conclusion
This study examined the demographic characteristics of rural skin cancer patients and the skin cancer diagnostic skills of GPs in South West Victoria. To our knowledge there is little existing research on this region of Australia specific to this topic. Results revealed males were significantly more likely to be diagnosed with skin cancer. This may be attributed to the occupational profile of the rural community and health-seeking behaviour of rural males. This study also found a lower NNT for melanoma and NMSC compared to existing Australian research, likely reflecting the increased pressure on rural GPs to diagnose skin cancer. Moreover, the diagnostic accuracy was demonstrated to increase with clinician experience and for clinicians with a special interest in skin cancer. However, there is limited large-scale research available for comparison. Given the vital role of GPs in skin cancer management, the diagnostic accuracy of Australian GPs should be further investigated.
References
You might also be interested in:
2014 - Healthcare use and prescription of opioids in rural residents with pain
2009 - Survey of a videoconference community of professional development for rural and urban nurses






