Introduction
Pregnancy increases the requirement for iodine by at least 50%, and a lack of iodine in the diet can result in maternal iodine deficiency. In the fetus1, iodine concentrations of less than 50 mg/L can increase concentrations of thyroid stimulating hormone (TSH) and the risk of goiter2. The causal relationship between iodine deficiency, goiter and congenital hypothyroidism has been described3. Maternal thyroxine crosses the placenta before the onset of fetal thyroid function (weeks 10–12) and accounts for 20–40% of maternal thyroxine measured in cord blood at birth. Thyroid hormones are necessary for neuronal migration and myelination; iodine deficiency irreversibly impairs fetal brain development and increases the risk of stillbirth, miscarriage and congenital anomalies4. Severe iodine deficiency results in maternal and fetal hypothyroidism and associated serious adverse health effects, including congenital hypothyroidism and growth retardation. Birth weight can be affected by slight variations in normal maternal thyroid hormone levels4-6. Excess iodine in the diet of pregnant women can induce factitious hyperthyroidism7. The World Health Organization (WHO) recognizes that in Colombia there is no iodine deficiency in the general population7,8. The few studies available in Latin America are limited to children in metropolitan areas and not to pregnant women in non-metropolitan areas. A descriptive cross-sectional analytical study was conducted to determine the prevalence of the above conditions in six non-metropolitan areas of Colombia.
Methods
Pregnant patients from Indigenous and Afro-descendant towns and institutions that provide health services of the departments of Amazonas, Cauca, Cesar, Córdoba, Guajira and Meta, who were insured in the General System of Social Security in Health, were invited to participate if a signed informed consent was evaluated. The protocol was reviewed and approved by the departmental governments through the departmental health secretariats and their Indigenous affairs offices. It was also submitted for consideration by the Indigenous communities and approved by the Indigenous governors of the respective ethnic groups. The number of patients in each territorial entity was proportional to the number of Indigenous and Afro-descendant insured pregnant women in the territory. The sample was calculated with a margin error of 5 percentage points and 80% power. The sample was overestimated by 10% because of the potential loss of patients and/or urine samples during river, land, or air transport. The pilot test was conducted in the municipalities of Northern Cauca (Corinto, Puerto Tejada and Santander de Quilichao) to determine the degree of understanding of the questions in the questionnaire and to establish the prevalence of iodine deficiency in the Indigenous and Afro-descendant populations. With these data, the sample was established for the Indigenous and Afro-descendant population of six non-metropolitan areas of Colombia in the other departments. Translators of native language to Spanish were used when necessary.
The pregnant patients were recruited during 2019 (January to August). A descriptive cross-sectional analytical study was conducted. Sociodemographic, clinical and thyroid palpation characteristics were collected by means of a precoded form and by patient interview and physical examination of the patient included in the study. Only data from fetal growth were obtained from patient medical history record; the remaining clinical data were obtained directly and face to face with the pregnant patient by means of physical medical examination. Inclusion criteria were pregnant women in prenatal control recognized by self as Indigenous or Afro-descendant, without pathologies triggered by pregnancy (preeclampsia, preterm delivery, gestational diabetes) and with a reliable gestational age according to previous obstetric ultrasound, whose residence was in a non-metropolitan area. The exclusion criteria were having underlying diseases involving a hypo-sodium diet (heart failure, chronic arterial hypertension, chronic renal failure) or under-treatment for chronic non-communicable diseases, or having previous thyroid pathologies or under-treatment for the same, or who had not freely accepted their participation in the study, or who ingested daily multivitamins with supplemented concentrations of iodine.
The dependent variable was the presence of goiter and intrauterine growth restriction (IUGR). The presence of goiter was established by clinical evaluation7. Only one experimentally trained researcher evaluated the presence of goiter in all pregnant women included in the study to reduce the interoperator bias in the clinical evaluation. Goiter level 1 was determined when the thyroid gland was palpable by hyperextension of the neck, goiter level 2 when the thyroid was palpable without hyperextension of the neck, and goiter level 3 when the goiter was visible without palpation3. IUGR was determined by ultrasonography (fetal growth < 10th percentile). The independent variables were the assessment of iodine levels in spontaneous urinary samples and iodine levels in sal de cocina (cooking salt) collected in the home of the pregnant women recruited in the study. Sociodemographic characteristics were evaluated according to the criteria of the National Administrative Department of Statistics (DANE) and their classification (very poor socioeconomic income, stratum 1; medium socioeconomic income, stratum 3; very high socioeconomic income, stratum 6). Social determinants were evaluated according to the National Survey of Health and Demography (ENDS 2015). The database was provided for research purposes by PROFAMILIA Colombia (non-government organization) Bogotá DC, Colombia.
The urine sample was collected under conditions of perineal cleanliness and good hydration of the pregnant woman. Subsequently, a physical evaluation of the thyroid gland was performed and, if goiter was found, it was classified according to the classification previously defined by WHO7. The laboratory tests were processed by the Nutrition Laboratory of the Faculty of Health of the Universidad del Valle. The confounder variables were the sociodemographic characteristics, drinking and smoking habits in the pregnant women recruited in the study.
Urine samples collected in rural Indigenous and Afro-descendant communities in non-metropolitan areas were preserved and kept in cold chain at –20ºC until analysis. The urine samples were delivered without identifying the patient by name, only by a code, depending on the site where they came from, with a consecutive assignment number (AZ, Amazonas; CE, Cesar; SQ, Cauca; MI, Córdoba; GU, Guajira; MT, Meta). The laboratory reported blind results only with the code, without knowing their origin. The technique used by the laboratory was the colorimetric method with arsenic ion, validated by WHO for this type of study9. For quality control purposes, it was established that the laboratory results would have a blind counter-sample analysis, processed at the Vírgen de la Arrixaca University Hospital of the University of Murcia (Murcia, Spain). The gestational age was placed within the gestational trimester (TI, between verification of pregnancy and week 13 of gestation; TII, between weeks 14 and 28 of gestation; TIII, between weeks 29 and 42 of gestation). Gestational age corresponded to weeks of amenorrhea correlated with early obstetric ultrasonography, when available. Analytic mass spectrophotometry was performed in the chemical laboratory of the Faculty of Sciences of the Universidad del Valle. All the study data were recorded in an Access database, checked and exported to the general database. Univariate and bivariate analyses between dependent variables and independent variables were performed. Odds ratios (OR) with a 95% confidence interval (CI) were calculated. A logistic regression was performed. A multiple correspondence analysis was performed to identify the geographical location of iodine deficiency by regions. To determine the statistical significance, a p-value <0.05 was considered significant.
Ethics approval
The protocol was approved by the institutional ethics committee of the Faculty of Health of Universidad del Valle in Cali, Colombia (Act 006-19R).
Results
A total of 318 Indigenous and Afro-descendant pregnant women were invited to participate in the study; 298 (94.3%) met the inclusion criteria and agreed to voluntarily sign the informed consent form. Four patients were excluded from the study because despite having voluntarily agreed to participate and having the objectives of the study explained to them, they refused to collect and submit the urine sample. Fourteen patients were excluded because urinary tract infection was detected with the sample collected and they were referred for medical consultation. Two patients were excluded due to the loss of cold chain in air transport. We analyzed the results of 298 patients. The delivery of the recruited pregnant women was before the pandemic emergency of COVID-19 (March 2020).
The mean age of the recruited pregnant women was 24 years (range 13–44 years). Sociodemographic characteristics of the pregnant patients (Table 1) showed that they were from poor geographic areas with low educational level. Twelve (4.0%) pregnant women had an ultrasound diagnosis of intrauterine growth restriction (IUGR), which was found in their clinical history. Five (40.8%) pregnant women with IUGR were from Cauca, three (25.0%) were from Córdoba, two (16.7%) were from Amazonas, one (8.3%) was from Meta, and one (8.3%) was from Guajira. Three women (1.0%) reported having consumed alcohol and none reported having smoked during their pregnancy.
A total of 69 (23.3%) pregnant women were observed to have goiter (38 (41.7%) living in the department of Cauca, 10 (35.7%) in Guajira, 5 (31.2%) in Meta, 6 (27.2%) in Amazonas, 10 (13.8%) in Córdoba (p=0.0001)). Of the urine samples, 41 (13.7%) were collected in the first trimester of gestation, 103 (34.6%) in the second trimester, and 154 (51.7%) in the third trimester. Of the 42 (14.9%) pregnant women observed to have an iodine deficiency (<100 µg/L), 41 (16.5%) were Indigenous, and 1 (2.0%) was Afro-descendant (p=0.04); 7 (5.1%) were severely iodine deficient (<20 µg/L), 19 (13.8%) were moderately iodine deficient (20–49 µg/L) and 16 (11.6%) were mildly iodine deficient (50–99 µg/L). A total of 31 (22.5%) pregnant women had high urinary iodine levels (300–399 µg/L) and 14 (10.1%) had very high (400–499 µg/L) urinary iodine levels.
Being literate was observed to be a protective factor against iodine deficiency (<100 µg/L) (OR=0.19, 95%CI 0.04–0.84, p=0.016). Illiteracy was more frequent with severe iodine deficiency (62%) than with moderate iodine deficiency (4%) and without iodine deficiency (20%). In the department of Cauca, it was observed that illiteracy and iodine deficiency (<100 µg/L) were associated with goiter (OR=6.72, 95%CI 3.9–9.5, p=0.038). The department of Amazonas was observed to have the highest frequency of severe iodine deficiency (Fig1).
The median of iodine levels in cooking salt was 14.91 ppm (range 3.95–62.02 ppm). Both Indigenous and Afro-descendant pregnant women had very low levels of iodine in cooking salt (median Indigenous: 11.97 ppm (range 3.91–39.04 ppm); median Afro-descendant pregnant women: 18.36 ppm (range 12.31–62.02 ppm)) without statistical differences by ethnic factor.
Table 1: Sociodemographic characteristics of the patients’ non-metropolitan geographic areas (2019)†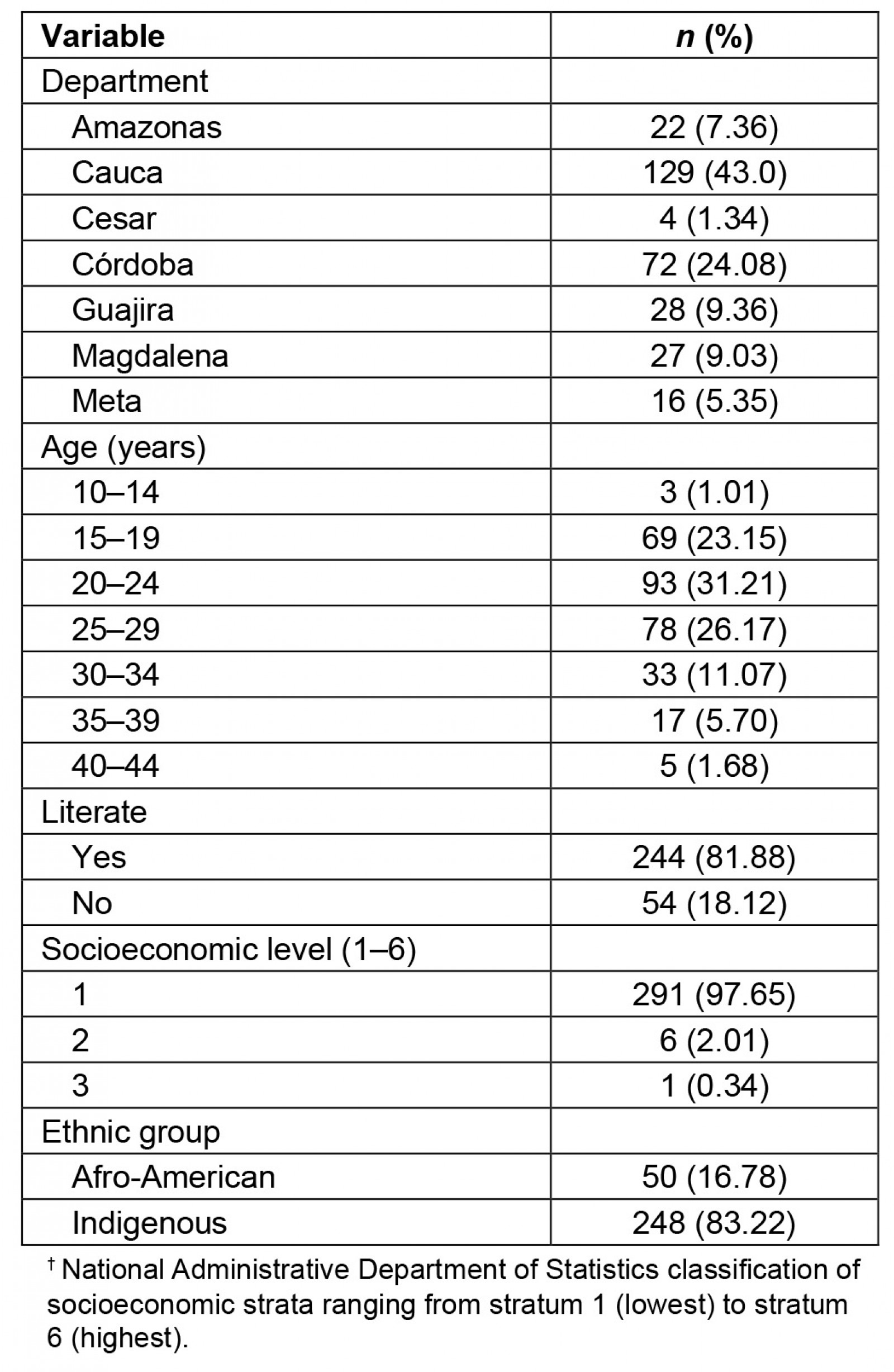
Discussion
Among the pregnant women, 42 (14.9%) were observed to have iodine deficiency (<100 µg/L), 69 (23.3%) had goiter, and 12 (4.0%) had an ultrasound diagnosis of IUGR. For pregnant women, a high incidence of goiter and iodine deficiency was observed. The relevance of the results is that there are no studies on pregnant women in Indigenous and Afro-descendant communities residing in non-metropolitan areas in Latin America. The common denominator in the non-metropolitan areas is unsatisfied basic needs, food insecurity, low socioeconomic level, illiteracy, poor communication routes, low socioeconomic level and low quality of life. This situation is similar in all non-metropolitan areas and it’s not a variable of confusion; all areas were comparable according to their socioeconomic characteristics (Table 2). Considering the adverse geographical conditions and the low educational level of the pregnant women included in this study, it was not possible to collect 24-hour urine samples in the fieldwork. However, the measurement of urinary iodine in spontaneous urine is a good indicator of recent iodine intake, since more than 90% of dietary iodine appears in urine over time and can be extrapolated to daily iodine intake. This quantification of iodine does not provide direct information about thyroid function; a low value indicates that a population is at increased risk of developing thyroid disorders. This evaluation is recommended by WHO/International Council for the Control of Iodine Deficiency Disorders/UNICEF to assess iodine nutrition in pregnant women as a community screening test7. According to the results of the present study, in the non-metropolitan area, the major proportion of severe iodine deficiency was in the department of Amazonas (Fig1). Severe iodine deficiency has potential risks for impaired cognitive, neurological, and growth and developmental function in children10-12. The department of Córdoba had the highest proportion of moderate iodine deficiency (20–49 µg/L). It is recognized that cognitive deficits are associated with iodine deficiency in children, which cannot be limited to severe deficiencies, given that mild to moderate iodine deficiency in pregnancy somewhat affects the cognitive function and intelligence of children, as reported by some observers13,14. Iodine deficiency is a risk for fetal brain development and brain growth and development in intrauterine life; however, iodine excess is also a risk, as it can lead to factitious hyperthyroidism. The analytic observational descriptive study design identified excess levels of urinary iodine levels in the department of Cauca when the urinary samples were obtained (Fig1); however, in the same department, a high prevalence of goiter and high risk of having goiter was observed, probably because in the past these pregnant women were exposed at least to chronic iodine deficiency with increases of TSH4.
Multiple correspondence analysis identified the department of Cauca as having the highest iodine levels (Fig1), which corresponds to the time of the study; however, it was also the department with the highest prevalence (41.7%) (p=0.0001) of goiter (sustained iodine levels <50 µg/L) (capable of increasing TSH). As is known, thyroid hormone in pregnancy not only facilitates neuronal connections but also favors fetal growth and development, a plausible concept with the results of this study, where the only department with high risk to goiter and with high incidence of intrauterine growth restriction was the department of Cauca. It was surprising to observe how iodine deficiency (<100 µg/L) was more frequent in the Indigenous pregnant women and almost absent in the Afro-descendant pregnant women, given that both ethnic groups resided in the same non-metropolitan populations. We believe that cultural factors in these ethnic groups could partly explain this situation.
According to the criteria of WHO, this study has identified a public health problem, not known before, since it has been said that there was no iodine deficiency in the country because salt is fortified with iodine. However, these studies were conducted in metropolitan areas of the country, where the situation is different from that observed in minority and vulnerable populations in non-metropolitan areas. WHO considers that a goiter prevalence higher than 5% in pregnant women in a community should be considered a public health emergency7, since iodine deficiency in pregnancy is the main cause of mental retardation and avoidable cerebral palsy7. In this study, we identified two problems probably related to the iodine deficiency in urinary samples and very low levels of iodine in cooking salt. Further studies are needed to clarify if these are independent problems, or if they are or are not correlated.
In developed countries such as Australia, for example, iodine levels have been observed to be lower in the Indigenous population than in the non-Indigenous population. When comparing iodine levels among pregnant women of the Indigenous population according to place of residence, lower levels were observed in those Indigenous people who resided in non-metropolitan areas, than in those who resided in metropolitan areas15, which is consistent with the results of the present study. It has been shown that infant survival improves when iodine deficiency is corrected before or during pregnancy, and neonatal mortality can be reduced by up to 65% when the population at risk is supplemented with iodine during pregnancy16. In countries where cooking salt is not fortified with iodine, the prevalence of congenital hypothyroidism in children assessed at 4 years is higher11-14. Preventive treatment of iodine deficiencies should be a goal as should avoiding high iodine levels in pregnancy17. One of the strengths of this study was that the pregnant women were evaluated in their territories with the approval of their Indigenous authorities. Second, all regions of the country were included in the study. The very low level of schooling and socioeconomic status of the population studied were limitations of the study; however, the social determinants are similar without differences17.
The date of the pregnancy was not reliable and access to an obstetric ultrasound was an important limitation; therefore, we used the evaluation of the uterine height to determine the trimester. The measurement of the fundal height is helpful only when it is a single fetus, when it is taken by trained health personnel, with the bladder evacuated, and ultrasound is not available17.
An analytical descriptive study does not allow the determination of causal relationships, since there is no temporality; it only allows the generation of hypotheses. The usefulness of the present study is that it identified a public health problem, not previously visualized, because previous studies of the prevalence of iodine deficiency were conducted mostly in metropolitan areas, where few patients from minority populations were included7,18. The effect of iodine deficiency during pregnancy and its long-term effects on cognitive and growth and developmental aspects need to be clarified in Indigenous pregnant women residing in non-metropolitan areas.
Table 2: Social determinants of health in the Indigenous and non-Indigenous populations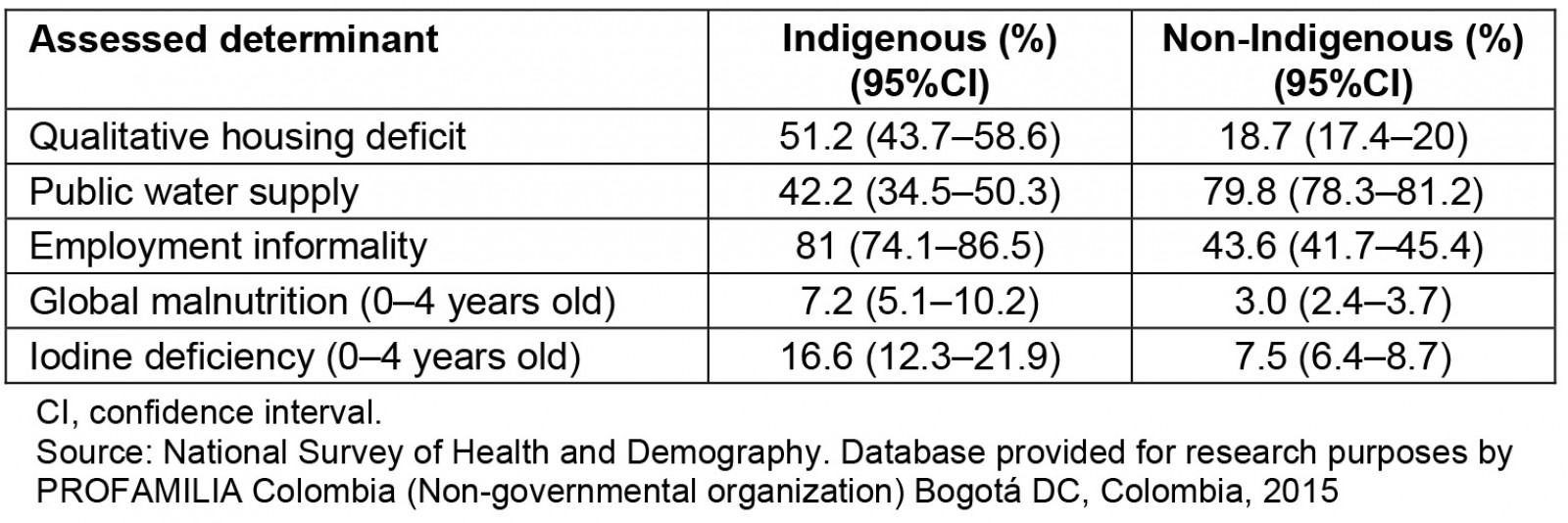
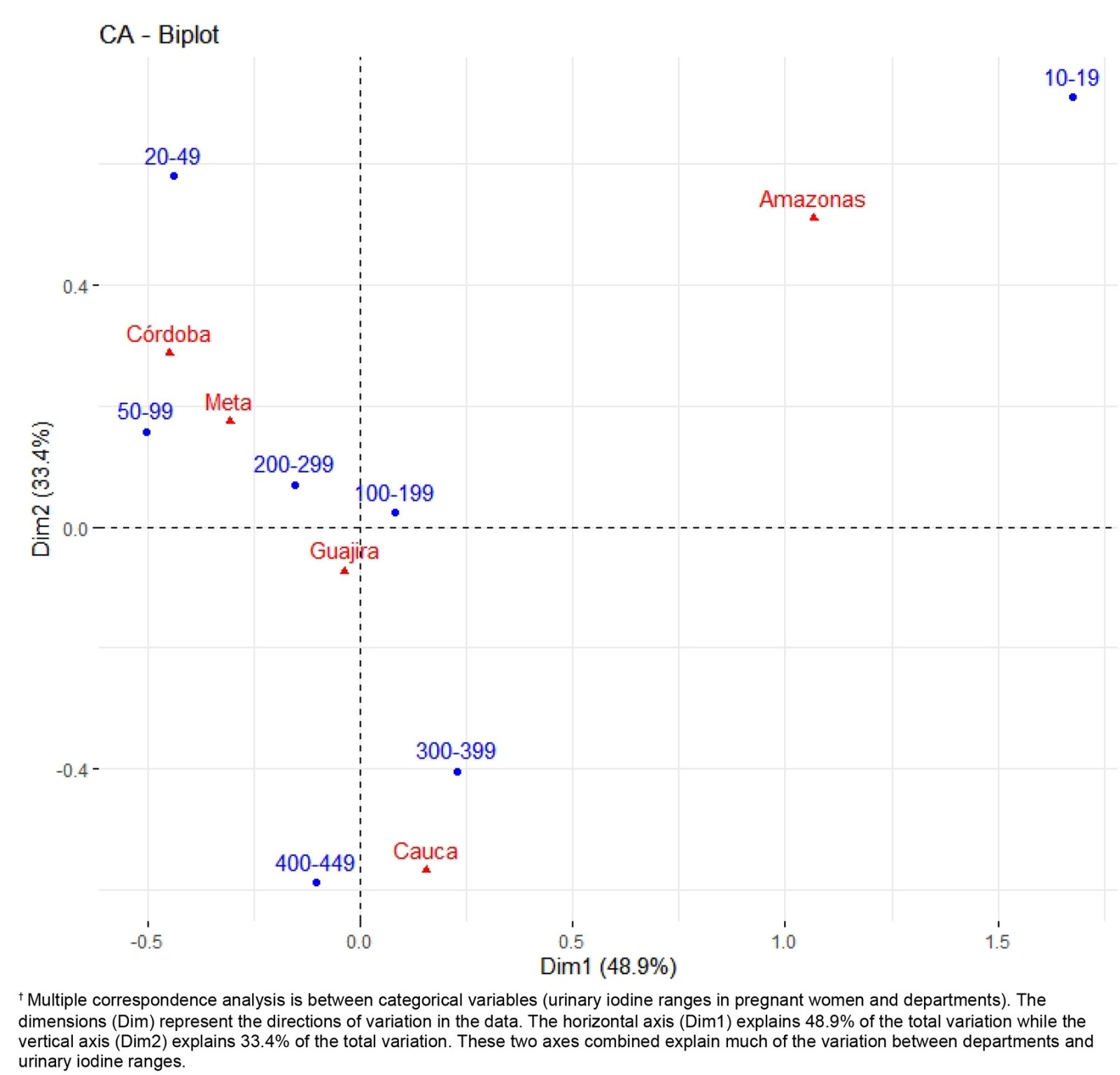 Figure 1: Multiple correspondence analysis between urinary iodine levels and non-metropolitan geographic areas (2019).†
Figure 1: Multiple correspondence analysis between urinary iodine levels and non-metropolitan geographic areas (2019).†
Conclusion
In pregnant women of minority ethnic groups of non-metropolitan areas of Colombia, a high prevalence of goiter, iodine deficiency and IUGR was observed, with regional differences. The department of Cauca was the only one with high risk of goiter.
Acknowledgements
The authors would like to thank the different governors’ offices, their departmental health secretariats, their Indigenous affairs offices, and the healthcare institutions of the different departments for their collaboration in the realization of this study. The authors would like to thank the statistician and epidemiologist Jennifer Murillo Alvarado for her contribution to the independent reporting of the results of this study.
Funding
Funding was received from the Universidad del Valle (Grant 1852-18).
Conflicts of interest
The authors declare that they have no institutional or personal interest in the objectives of the research or in the development of the project.













